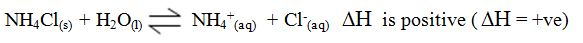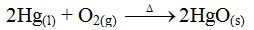THE CHEMICAL KINETICS, ENERGETICS AND EQUILIBRIUM
CHEMICAL KINETICS.
Chemical kinetics refers to the study of the rate of chemical reactions. It includes analysis of conditions that affect speed of a chemical reaction, understanding reaction mechanisms and transition states and forming mathematical models to predict and describe a chemical reaction.
Chemical reaction
Chemical kinetics may also be called reaction kinetics or simply "kinetics".
THE RATE OF CHEMICAL REACTION
Chemical reactions take place at different rates. Some are fast whereas others are very slow. Let us consider the following reactions:
For instance, Addition of sodium metal to water:
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
The reaction takes place immediately and violently. It is therefore a fast reaction.
OR
The rusting of iron in the presence of air and water giving hydrated iron (III) oxide, F2O3.XH2O: This is an extremely slow reaction
In general, the rate of a chemical reaction is determined by measuring the amount of reactant used up per unit of time or the amount of product produced per unit of time.
In general, the rate of a chemical reaction is determined by measuring the amount of reactant used up per unit of time or the amount of product produced per unit of time.
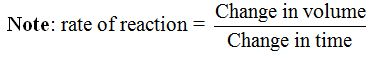
Therefore, the rate of a chemical reaction simply refers to the amount of reaction which occurs in a unit time.
COLLISION THEORY
is used to predict the rates of chemical reactions, particularly for gases.
The collision theory is based on the assumption that for a reaction to occur it is necessary for the reacting species (atom or molecules) to come together or collide with one another.
Effective collision.
Not all collisions, however, bring about chemical change.
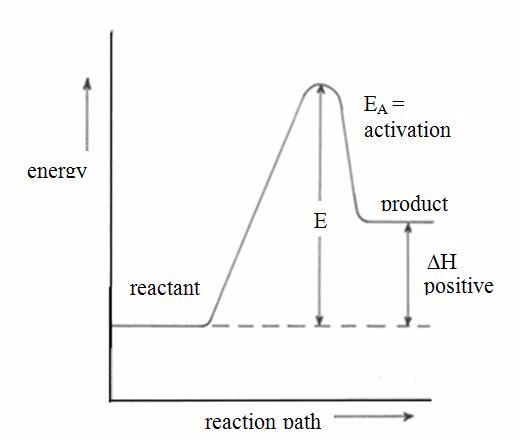
A collision will be effective in producing chemical change only if the species brought together possess a certain minimum value of internal energy equals to activation energy of the reaction.
Furthermore, the colliding species must be oriented in a manner favorable to the necessary rearrangement of atoms and electrons. Thus, according to the collision theory, the rate at which a chemical reaction proceeds is equal to the frequency of collisions.
Activation Energy.
This is the minimum amount of energy required for a chemical reaction to take place. OR is the energy which must be provided to the reactants in a chemical reaction for them to reach an activated state from which the products can be formed.
The Concept of Endothermic and Exothermic Reactions
You
have met many different chemical reactions so far in chemistry. But
they all have one thing in common, that is, they involve an energy
change. The great majority of chemical reactions are accompanied by a
marked heat change.
During
chemical reactions as reactants form products, there is a change in
heat content. This is referred to as the enthalpy changeand is always
expressed in kilojoules per mole (kJmol-1). Two types of heat
change are distinguished. Those reactions that are accompanied by
evolution of heat to the surroundings are termed as exothermic reactions while those that are accompanied by absorption of heat from the surroundings are endothermic reactions.
- An exothermic reaction is one during which heat is liberated to the surroundings.
- An endothermic reaction is one during which heat is absorbed from the surroundings
When magnesium is burnt in air heat is evolved.
2Mg(s) + O2(g) → 2MgO(s)+ heat
The same case applies to the burning of coal in air.
C(s)+ O2(g) → CO2(g) + heat
Mixing sulphur nitrate and sodium chloride solutions gives a white precipitate of silver chloride and a temperature rise.
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq)
When
ammonium nitrate is dissolved in water, there is a fall in temperature.
Also adding a mixture of citric acid and sodium bicarbonate to water
produces bubbles and a fall in temperature. In both reactions, the
temperature of the water falls because the reactions take heat energy
from it. These reactions are therefore endothermic.
The
heat changes that occur during any chemical reaction represent changes
in the energy content of the whole system. The energy content may
increase or decrease depending upon whether heat is absorbed or evolved.
Energy Level Diagrams for Exothermic and Endothermic Reactions
For exothermic reactions,
the enthalpy changeis conventionally assigned a negative value. For
example, when pellets of sodium hydroxide or concentrated sulphuric acid
dissolve in water, heat is evolved and the system loses heat to the
surrounding.
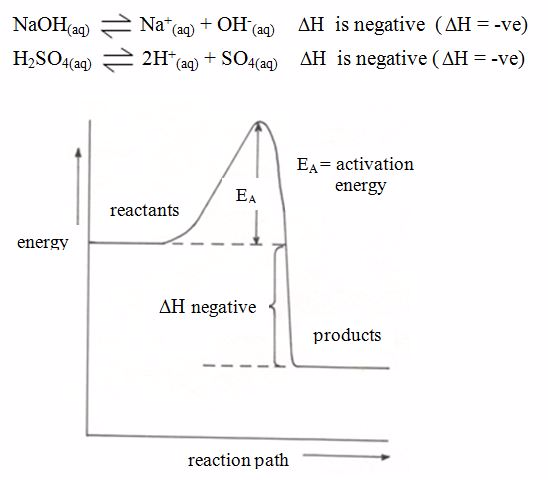
Energy level diagram for exothermic reaction
For endothermic reactions,
the enthalpy changeis assigned a positive value. For example, when
potassium iodide or ammonium chloride dissolves in water, heat is
absorbed from the surroundings.