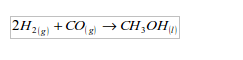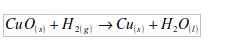HYDROGEN
Hydrogen is the highest and common and element in the universe. I form about 90% of the total mass of the universe. It is believed that the sun composes almost of hydrogen and helium. Hydrogen occur naturally in air as hydrogen gas. It also occur in combined state in water, petroleum, acid and natural gas and in almost all organic substances such as protein, carbohydrates and lipids just few to mention.
Uses of Hydrogen
Uses of Hydrogen Gas
1.
It is used in the manufacture of ammonia by the Haber process, which is
based on the direct combination of hydrogen and nitrogen.
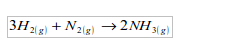
2. It is used in the hardening of vegetable oils to make margarine.
3.
It was formerly used for inflating balloons and air ships. But hydrogen
is inflammable and many accidents occurred. Its use has been replaced
by helium (another gas occurring in air). Nowadays, hydrogen is used by
meteorologists to fill weather balloons, which carry weather instruments
that record information on various elements of weather in the upper
atmosphere.
4.
It is used to prepare water gas, which is used as a fuel for space
rockets. When hydrogen contained in water gas is burned in air, it
produces extremely high heat energy that is used to power rocket
engines.
5.
It is used in welding by the atomic hydrogen torch. The complete
combustion between hydrogen and oxygen is a highly exothermic reaction
and can produce an oxy-hydrogen flame that has a temperature of nearly
2000ºC, and is therefore useful in the welding and cutting of metals.
However, the explosive nature of the combustion of hydrogen with oxygen
makes the use of oxy-hydrogen flame less favourable than the
oxyacetylene flame.
6.
It is used in the synthesis of hydrochloric acid. In this case,
hydrogen combines directly with chlorine to form hydrogen chloride gas.
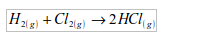
The hydrogen chloride gas is then dissolved in water to form hydrochloric acid.
7. It is used in the manufacture of methanol (wood spirit). In this process hydrogen combines directly with carbon monoxide.