IONIC THEORY AND ELECTROLYSIS
INTRODUCTION:
Some solid substances such as metals allow the passage of current electricity through them. Such materials are called conductors. molten salts and some solutions are also conductors of electricity.
Substances that do not allow current electricity to pass through them are called insulators or non conductors. Example of insulator includes rubber and wood.
Poor conductor is a substance that allow only small amount of electric current to pass through them. Examples of poor conductor of electric current is water.
Conductors have electrically charged particles that are movable. The movement of charged particles makes it possible for the materials (conductors) to conduct electric current. The negatively charged particles (electrons) help in conduction of electric current in different materials.
Electrolysis
has several uses in industry. Its main application has been in the
fields of manufacture of chemicals and in the purification of metals for
which other purification methods prove either too difficult or highly
expensive to apply. Some applications of electrolysis are as discussed
below:
The Industrial Purification of Copper by Electrolysis
Some
metals can be purified by means of electrolysis. This process is used
in industry to purify copper, which must be very pure 99.9% for
electrical wiring. Copper made by roasting the sulphide ore is about
99.5% pure (so it has an impurity level of 0.5%). This level of impurity
cuts down
electrical conductivity significantly.
electrical conductivity significantly.
This
is how the electrolytic purification (refining) process is carried
out:The anode is made of a large block of impure copper. The cathode is a
thin sheet of pure copper. The electrolyte is copper (II) sulphate
solution.During the refining process, the copper atoms of the impure
block become ions (the anode dissolves).Cu → Cu2+ + 2e-
The ions from the solution become atoms.
Cu2+ + 2e- → Cu(s)
They
stick onto the cathode. A layer of pure copper builds up on the
cathode. As electrolysis takes place, the cathode gains mass as copper
is deposited on it. As a result, the cathode gets smaller while the
cathode gets bigger as electrolysis proceeds. Eventually the whole
cathode dissolves.
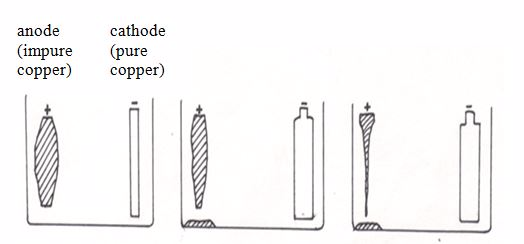
Purification of copper by electrolysis
Only
pure copper sticks to the cathode. Most impurities fall to the bottom
of the electrolytic cell. They form a solid material (anode sludge or slime)
which contains small quantities of precious metals such as silver, gold
and platinum. The precious metals recovered from the slime are purified
and sold.
An Experiment on Electroplating of Metallic Materials
Carry out an experiment on electroplating of metallic materials
Electroplating
is the coating of a metal with a layer of another metal by means of
electrolysis. Electrolysis can be used to coat a thin layer of a less
reactive metal onto a more reactive metal. The thin layer of less
reactive metal will provide protection from corrosion for the more
reactive metal underneath. It may also make the product more attractive.
The
object to be coated should be made the cathode and the coating material
should be the electrolyte. The most commonly used metals for
electroplating are copper, chromium, silver and tin.
Steel
can be electroplated with chromium or tin. This prevents the steel from
rusting and gives it a shiny, silver finish. This is also the idea
behind chromium-plating articles such as car bumpers, kettles, bath
taps, etc. Chromium does not corrode, it is a hard metal that resists
scratching and wear, and can also be polished to give an attractive
finish.
Nickel
can be electroplated with silver. This will make nickel more
attractive.The diagram below shows how a steel jug is electroplated with
silver. The jug becomes the cathode of an electrolytic cell. The anode
is made of silver. The electrolyte is a solution of a silver compound,
for example silver nitrate.
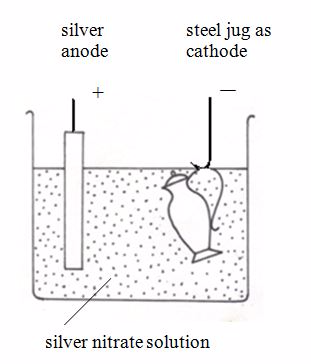
Silverplating a steel jug
At the anode: The silver dissolves, forming ions in solution:Ag → Ag+ + e-
At the cathode: The silver ions receive electrons, forming a coat of silver on the jug:Ag+ + e-→Ag (s)
When the layer of silver is thick enough, the jug is removed.In general, to electroplate any object with metal M, the set up is:
- Cathode – object to be electroplated
- Anode – metal M
- Electrolyte – solution of a soluble compound of M