Concept of Pollution
The Concept of Pollution
Pollution
can also be defined as the introduction of contaminants or pollutants
into the natural environment. The environment comprises of living
(biotic) and non-living (abiotic) things such as plants, animals, air,
land and water.
Contaminants
or pollutants are harmful substances introduced into the environment
that disturb the balance of nature. Pollution can be in the form of
chemical substances or energy such as noise, heat or light.
Human activities play a major role in the pollution of the environment. Humans engage in a myriad of activities such as agriculture, manufacturing, transport, waste disposal, mining etc. All of these activities contribute to environmental pollution in one way or another.
There are three main types of pollution:
- Terrestrial (land) pollution
- Aquatic (water) pollution
- Aerial (air) pollution
Terrestrial Pollution
The Concept of Terrestrial Pollution
Terrestrial
pollution is the degradation or destruction of the earth‟s surface and
soil, directly or indirectly, as a result of human activities. The human
activities refer to any activities performed by man that lessens the
quality and or productivity of the land as an ideal resource for
agriculture, forestation, construction, etc.
Human Activities which Cause Terrestrial Pollution
The
human activities responsible for this kind of pollution include poor
agricultural practices, mining, waste dumping and urban waste disposal.
Below are some causes of terrestrial pollution.
Agricultural activities
Because
of the ever-increasing human population, demand for food has increased
rapidly. Farmers often use fertilizers to increase crop production and
pesticides to get rid of pests, fungi and bacteria that destroy the
crops or harm animals. The overuse of such agrochemicals results in the
contamination and poisoning of the soil. Other causes of soil pollution
from agricultural activities include:
- poor methods of irrigation which causes the leaching of cations down the soil surface;
- manure heaped on land, which may leach down the soil; and
- oil spillages that seep into the soil.
Mining activities
During
mineral extraction, several land spaces are created beneath the soil
surface. The underground holes dug during mining causes the land to sag
or subside (caving in). This is nothing but the nature‟s way of filling
the spaces left out after mining or extraction activities. This destroys
the land and makes it unfit for use.
Mining involves the use of toxic chemicals used for mineral separation. When these chemicals drain into the soil, it gets polluted.
Rain
water often leaches harmful substances from the exposed mining waste
into the ground. These harmful substances are like heavy metals (e.g.
arsenic) and sulphuric acid or chemicals used in processing the ores
(e.g. cyanide. These chemicals are another cause of soil contamination.
Deforestation and soil erosion
Deforestation
refers to indiscrimate cutting of trees in search of land for
agriculture, settlement, mining, industrialization etc. Trees have got a
number of advantages which include attracting rainfall, checking soil
erosion and breaking strong winds. The act of cutting down trees
carelessly leaves the land bare and hence exposes it to agents of
erosion.
Soil
erosion causes land pollution. The eroded soil loses its nutrients and
organic matter, as well as its ability to hold water. As such, soil
erosion can render a fertile land as no longer suited for agriculture,
or even turn originally fertile lands into barren deserts.
Other
than causing soil erosion, deforestation has also been linked to floods
which can, in turn, be seen as another cause of land and water
pollution.
Sewage disposal
Sewage
refers to a waste, in solution or suspension, carried off in sewers or
drains with intention of removing it from the community.
In
areas where there are no water bodies into which to dump urban sewage
directly, it is dumped into sewage pools that are usually dug far away
from residential areas to avoid bad smell emitted by the deposited
sewage.
The toxins and poisonous chemicals in the sewage gradually seep down into the ground, thus polluting the land and killing beneficial soil microorganisms.

Garbage disposal
Tonnes
of garbage are produced each year, especially in urban and industrial
areas. Garbage is collected and moved to the dumping sites allocated for
that purpose.
The
garbage in dumping sites comprises of biodegradable and
non-biodegradable wastes. The biodegradable wastes are those that can be
broken down easily by the action of microorganisms,e.g. bacteria and
fungi. The degradable waste matter may include materials such as rotten
foods, kitchen wastes etc.
The non-biodegradable wastes are those that cannot be decomposed by microorganisms and thus remain on the ground for a long time. The non-biodegradable wastes are such as plastic, polythene bags, metal, some clothing and glass.
One
of the common causes of land pollution in dumping sites is the
contamination of the soil with toxic and even hazardous substances.
Needless to say, the ability of this soil to support life is
significantly affected.
Transfer of toxic wastes
Industries
in developed countries produce much toxic wastes, including the deadly,
reactive nuclear wastes. However, these countries have very strict laws
which prohibit the dumping of such toxic wastes in their countries. The
easiest and cheapest alternative is to dump them in the developing
third-world countries. This is because of the greedy and selfish leaders
in poor countries are easily that agree to sign contracts to allow the
disposal of such dangerous wastes on their lands for their own economic
gains.
Hazards Caused by Terrestrial Pollution
The
extent of terrestrial pollution is often overlooked because its effects
are not well evident to most people. However, land pollution has got a
number of negative effects to soil, soil organisms, man, plants and
animals. The following are some effects of terrestrial pollution:
- Wastes dumped carelessly can endanger the health of man as well as other organisms. Broken glass, metal and other sharp objects may pierce one‟s skin and introduce disease germs into the body. Empty cans, glass and plastic containers are potential breeding grounds for mosquitoes which spread malaria and other diseases. Rotten organic matter may harbour many disease germs and they also produce noxious smell when they rot. The rotten wastes also attract flies which transmit a number of enteric diseases like dysentery, cholera, diarrhoea, etc.
- Land pollution causes chemical contamination to the ecosystem. This occurs when the chemicals in the waste matter poison the soil. Then plants growing on the poisoned soil, animals that eat these plants and even humans are all affected by these chemicals. This process is called biomagnifications and is a serious threat to the ecology. It can lead to the loss of some types of plants and animal life as well as create long-term health problems such as cancer and other deformities in humans. Radiation from nuclear wastes causes healthy problems such as cancers and other deformities.
- Piles of waste in urban areas keep growing due to increase in waste. When this waste is burned it produces a lot of smoke that leads to air pollution.
- Soil erosion (as a form of land pollution) leads to loss of land for agriculture, settlement, forest cover, fodder patches for grazing, etc.
- Land pollution leads to loss of ecosystem and hence directly or indirectly cause change in climatic patterns.
- Deforestation causes imbalance in the rain cycle. A disturbed rain cycle affects a lot of factors such as reduction in the green cover. Plants help absorb excess carbon dioxide from the air and release oxygen to the atmosphere. This process helps to balance the atmosphere. Without vegetation cover, excessive accumulation of carbon dioxide in the atmosphere causesconcerns like global warming, the greenhouse effect, irregular rainfall, and floods among other imbalances.
- Land pollution damages terrestrial life, especially plants. This greatly affects wildlife and other animal species which are forced to move further away and adapt to new regions or die trying to adjust.
- Heaps of different wastes from mining activities make the environment unsightly and ugly.
- Terrestrial pollution is a big problem in urban areas where waste production outweighs waste disposal. In such areas you find poor and blocked sewage system, effluent from domestic toilets flowing on the streets and roads, and dirty water carelessly poured on the ground. This makes life in urban areas uncomfortable and a mere nuisance.
Different Methods of Preventing Terrestrial Pollution
The following are some measures that can be taken to prevent and control land pollution:
Recycling and reuse
Recycling
is the processing of changing used materials into usable raw materials
instead of discarding them as wastes altogether. Scrap metals, plastic
bottles and glass should be recycled instead of being dumped into the
environment. Packaging materials such as plastic bags, beverage and
water bottles can be recycled or re-used for packaging or carrying
goods.

Using biodegradable materials
Emphasis
should be put on manufacturing and using materials that can easily be
broken down (biodegradable). For example, paper bags should be
manufactured and used instead of plastic (polythene) bags, which are
non-biodegradable. Biodegradable plastics have been developed and are
used. Some biodegradable plastics include
- biopolymers such as those used in making surgical sutures;
- photodegradable plastics, which break down upon exposure to light: and
- soluble plastics which can be broken down by water
Proper disposal of wastes
Urban
waste should only be dumped in allocated dumping sites. These sites
must be far away from residential areas to avoid the risk of spreading
diseases, pest infestation and a bad smell. The sewage should be
properly treated before being drained into dumping pools. Some of the
methods that can be used to curb urban waste problem include
incineration and recycling. Paraffin should be poured onto the sewage
pools to suffocate and kill mosquito larvae and hence prevent the spread
of malaria to residents living in the vicinity of the dumping pools.

Reducing the use of agrochemicals
Farmers
should be advised and encouraged to avoid dependency on agricultural
chemicals (fertilizers, herbicides pesticides, etc). All of these
chemicals pollute the soil and affect soil microorganisms a great deal.
Farmers should use organic manures and chemicals in their agricultural
operations. These do not pollute the land or affect crops and animals as
compared to artificial chemicals. They help to improve soil structure
and hence prevent soil erosion.
Making and enacting environmental laws and policies
The
government should make and implement laws and regulations to prevent
and control terrestrial pollution. Likewise, local government
authorities should make by-laws aiming at curbing the problem of
environmental pollution. The laws must clearly state guidelines and
procedures to be followed by everybody regarding environment sanitation.
These may include:
- discharge and treatment of sewage;
- disposal of lethal nuclear wastes;
- use of agrochemicals in agricultural production;
- use of plastic and related materials;
- disposal of toxic chemicals and solid wastes from industries; and
- careless littering of the environment by irresponsible people.
Creating public awareness
The
general public should be educated about the importance of keeping the
environment clean and the benefit of living in a clean environment. This
knowledge can be conveyed through meetings or via mass media such as
television, radio and newspapers. It may also be conveyed via
announcements, posters and other social media.
Aquatic Pollution
The Concept of Aquatic Pollution
Aquatic
pollution is the introduction of substances that lower the quality of
water into water bodies such as oceans, rivers, lakes, aquifers and
ground water. This makes the water unsafe for use in homes and
industries. Water pollution also affects living organisms (plants and
animals) living in water.
Human Activities which Cause Water Pollution
There are two broad categories of sources of water pollution namely, point and non-point sources.
A
point source is one that delivers harmful substances directly into a
water body. An example of a point source of water pollution is a pipe
from an industrial facility discharging effluent (liquid waste) directly
into a river, lake or sea.
A
non-point source of water pollution is a source that delivers
pollutants into the water body indirectly through transport or
environmental change. An example of a non-point source of water
pollution is when fertilizer from a farm field is carried into a stream
by rain (surface run off).
The following are some of the major causes of water pollution:
Pesticides
Pesticides
that are applied to crops and animals drip onto the soil and may
eventually run off into the local streams and rivers. They can also seep
down to reach ground water. This contaminates the water and makes it
unwholesome for human use and can drastically affect the aquatic and
other organisms whose lives depend on that water.
Fertilizer (nutrient pollution)
Many
pollutants, including sewage, manure and chemical fertilizers, contain
nutrients such as nitrates and phosphates. Nitrates are very soluble.
Rain washes or leaches them out of the soil into rivers. In the rivers,
excess levels of nutrients (the nitrates) stimulate the growth of
aquatic plants and algae. These form a layer on the water surface. A
layer of these algae and aquatic plants on the surface of water will
prevent light and oxygen from reaching the organisms under the water. As
a result, these organisms will begin to die. When the aquatic organisms
and the algae die, bacteria feed on the remains. In the process, they
use up the oxygen dissolved in the water. Thus, the amount of oxygen in
water drops. As a result, fish and other river life die from oxygen
starvation, and the river becomes chocked and lifeless. This is called eutrophication. Water with limited dissolved oxygen supports only a few aquatic organisms. Such areas are called dead zones.
Oil spills
Oil
spills in oceans and seas cause water pollution and big problems for
local wildlife, fishermen and aquatic organisms. Oil spilled onto land
is also carried into water bodies by surface run off. This includes
drips of oil, fuel and fluid from motor vehicles, oil spilled onto the
ground at filling stations; and drips of oil from industrial machinery.
These sources and many more combine together to form continual petroleum
pollution to all of the world‟s waters.
Oil
spilled by ships, discharge of oily wastes, and drips from motor boats
are all significant sources of marine pollution. Drilling and extraction
operations for oil and gas can also contaminate coastal waters and
ground water.
Mining
Mining causes pollution in a number of ways. They include the following:
- The mining process exposes heavy metals and sulphur compounds that were previously locked deep in the earth. Rain water leaches these compounds out of the exposed earth, resulting in “acid mine drainage” and heavy metal pollution that can continue long after the mining operations have practically ceased.
- The action of rain water on piles of mining waste (tailings) transfers pollution to freshwater supplies.
- In gold mining, cyanide is intentionally poured on piles of mined rock (a leach heap) to chemically extract the gold from the ore. Some of the cyanide ultimately finds its way into nearby water.
- Huge pools of mining waste slurry (semi-liquid mixture) are often stored behind containment dams. If a dam leaks or bursts, water pollution is likely to take place.
- Mining companies in developing countries sometimes dump mining waste directly into rivers or other water bodies as a method of disposal.
Sediment
The
act of clearing the forests to get ample land for agriculture,
settlement or wood leaves the land bare and exposed to the agents of
denudation. This accelerates soil erosion and the sediment is free to
run into nearby streams, rivers and lakes. The increased amount of
sediment running off the land into nearby water bodies seriously affects
the fish and other aquatic life. Poor farming practices and cultivation
along and close to the rivers, exposes the soil to erosion agents. Soil
erosion causes water pollution.
Industrial processes
Most
of the water that is used in the production process in industries is
eventually discharged into water bodies. This waste water may contain
harmful chemicals such as acids, alkalis, salts, toxic chemicals, oil,
heavy metals and even harmful bacteria, and other reagents. These
substances affect the quality of water and the lives of aquatic
organisms.
In some cases, the waste water discharged into a water body may be hot enough to kill any organism living in that water.
Sewage
In
developing countries about 90% of untreated sewage is discharged
directly into rivers and streams. This renders the water unwholesome for
domestic and other uses. Untreated sewage harbours a myriad of
disease-causing organisms. This is the reason why diseases such as
cholera, dysentery, typhoid and bilharzias are very common among African
countries. Leaking septic tanks and other sources of sewage can
contaminate ground and stream waters as well.
Marine debris (marine litter)
Marine
debris is trash in the ocean. This is litter that ends up in ocean,
seas or other large water bodies. The debris mainly comes from urban
sewers and garbage thrown overboard from ships and boats.
Examples
of marine debris include plastic bags, water bottles, balloons, shoes,
lags etc. It can also include items that wash in from the ocean, such as
fishing line, ropes, nets and traps, and items from ship such as lost
cargo from container ships.

Air pollution
Air
pollution contributes substantially to water pollution. Pollutants like
mercury, sulphur dioxide, nitrogen oxides, and ammonia can get into the
water bodies from the air. This can cause problems like mercury
contamination in fish, acidification of lakes and eutrophication
(nutrient pollution).
Most
of the air pollution that affects water comes from coal-fired power
plants, vehicle exhaust fumes, and industrial emissions.
According to a long-term study by the United Nations Environment Programme (UNEP), it has been observed that the earth‟s oceans have absorbed enough carbon dioxide from the air to cause a slight increase in ocean acidification. Most of the carbon dioxide gas that acidifies the ocean results from human activities (man-made carbon dioxide). It is feared that further carbon dioxide uptake will increase acidification even more and cause the carbonate structure of corals, algae and marine plankton to dissolve. This could have negative impacts on the biological systems of the oceans
Heat
Heat
is a water pollutant. Increase in water temperature results in deaths
of many aquatic organisms. This is because, as water temperature
increases, the amount of oxygen that can dissolve in it also decreases.
Therefore, warm and shallow water will contain very little oxygen to an
extent that the dissolved gas will not sustain aquatic life.
These
increase in temperature is most often caused by discharge of cooling
water (which is always hot) by factories and power plants.
Global warming also contributes significantly to heating of the oceans.
Noise
Many
marine organisms, including mammals, sea turtles and fish, use sound to
communicate, navigate and hunt. Noise from ship engines and sonars has a
negative effect on these organisms. Following noise pollution, some
species may find it hard to hunt or detect predators. Others may not be
able to navigate properly.
The Hazards which are Caused by Water Pollution
The following are some of the effects of water pollution:
Waterborne diseases
Human
infectious diseases are among the most serious effects of water
pollution. Waterborne diseases occur when parasites or other
disease-causing microorganisms are transmitted via contaminated water.
Water may be contaminated by pathogens originating from excreta.
Waterborne diseases include typhoid, diarrhoea, dysentery, cholera,
bilharzia and many other diseases caused by bacteria, protozoa and
viruses. Contaminated water also spread intestinal parasites such as
hook worm, round worm, and ascaris.
Polluted
beach water can cause skin rashes, ear aches, pink eye, respiratory
infection, hepatitis, diarrhoea, vomiting and stomach ache.
Nutrient pollution
Nutrient
pollution stimulates the overgrowths of algae and other aquatic plants.
Algae can have direct toxic effects and finally result in oxygen
deficiency in water.
Certain
species of algae are toxic. Overgrowths of these algae result in
harmful algal blooms. These are more colloquiallyreferred to as red
tides or brown tides. Zooplankton eat the toxic algae. When fish eat the
zooplankton, the toxins are passed to fish. Ultimately, when fish is
eaten by seabirds, marine mammals and man, the toxins pass to these
organisms. In this way, the toxins pass to the food chain and become
part of it.
Blooms
of algae and seaweed also reduce water clarity. This makes it hard for
marine animals to find food. Algal bloom also blocks the sunlight needed
by sea plants, which serve as nurseries for many important fish
species.
When the algal overgrowths finally die, bacteria do feed on the remains. In the process, bacteria use up the oxygen dissolved in the water. In some cases, the process takes enough oxygen from the water such that oxygen level in the water falls too low to support normal aquatic life. As a result, fish and other aquatic organisms die from suffocation. The region becomes a coastal dead zone.
Some
algae are poisonous to fish and humans. People swimming through these
algae or swallowing the water may get rashes, eye irritation, muscle
pains, vomiting and diarrhoea.
Excess
nitrate in the river may get into drinking water. It increases the risk
of blue-baby syndrome. What happens is that bacteria in a feeding
bottle or a baby‟s body convert the nitrate to nitrite. This gets taken
up instead oxygen, by the haemoglobin in blood. The baby turns blue and
can die.
Nutrient pollution can also trigger unusual outbreaks of fish diseases.
Chemical contamination
Every
year many types of chemicals are draining into water. Severe chemical
spills and leaks into water bodies kill fish and other aquatic life.
Chemical pollution is caused by a number of toxic chemicals. The
following are some categories of water pollution effects due to chemical
contamination:
Every
year many types of chemicals are draining into water. Severe chemical
spills and leaks into water bodies kill fish andother aquatic life.
Chemical pollution is caused by a number of toxic chemicals.
The following are some categories of water pollution effects due to chemical contamination:
Pesticides
Pesticides
are carried in rain water run off from sources such as farm fields,
suburban lawns and home gardens into the nearest rivers and streams.
Pesticides
contained in drinking water and in the food chain can result in damage
to the nervous system, reproductive and endocrine systems and the liver.
It can also damage the DNA and cause various cancers.
Oil spills
Exposure to oil or its constituent chemicals can alter the ecology of aquatic habitats and the physiology of marine organisms.
The
oil (or chemical component of the oil) can seep into marsh and
sub-tidal sediments and remain there for many years. This negatively
affects marsh grasses, marine worms, and other aquatic life forms that
live in, on or near the sediment.
Compounds
of crude oil, called the polycyclic aromatic hydrocarbons (PAHs), can
remain in the marine environment for many years and are toxic to marine
life at even low concentrations. Prolonged exposure to PAHs can affect
development of marine organisms, increase susceptibility to disease, and
distort normal reproductive cycles in many marine species.
Mercury
Mercury
gets into water from coal-fired power plants, gold mining and some
other industrial processes. In the water, the elemental mercury is
converted to methylmercury, [CH3Hg]+, by certain bacteria. Then this new
form of mercury moves up the food chain of fish when small fishes are
eaten by a big fish. In the end, the big fish may be eaten by man and
the mercury is passed on to him.
The effects of mercury on humans are many and are already pretty well understood:
- Young children and unborn babies are at a higher risk because their body systems are still developing. Exposure to mercury in unborn babies can cause neurological problems such as slower reflexes, learning deficit, delayed or incomplete mental development, autism, and brain damage.
- Mercury can also cause serious nervous system problems in adults. These problems include Parkinson‟s disease, multiple sclerosis, and Alzheimer‟s disease. It can also cause heart disease and damage to the brain.
Industrial chemicals
Chemicals
used in industrial processes are being discharged into water bodies
daily. Many chemicals can have direct toxic effects on aquatic life.
Industrial spills into rivers kill fish for many kilometres downstream.

A
new threat from chemicals is the hormone-disturbing character of many
chemicals. The effects of hormone-disturbing chemicals include
interrupted sexual development, thyroid system disorders, inability to
breed, reduced immune response, and abnormal and parenting behaviour.
In
humans, endocrine disruptors lead to low immune function, mental
impairment, decreased fertility and increase in some types of cancers.
Mining
There is a good number of negative water-pollution effects from mining operations. They include the following:
Acid Mine Drainage (AMD):
This
refers to the out flow of acid water from metal mines or coal mines.
This is how it is formed: Mining process exposes rocks and soil. When
rain or surface water flows over exposed rock and soil, it combines with
naturally-occurring sulphur to form sulphuric acid. The acidified water
eventually finds its way to streams and ground water. This pollutes the
water and affects the local aquatic life. Some streams are so acidic
that they destroy the aquatic ecosystem completely.

Spills and leaks:
Leaks
in containment system, cyanide leach heap or breakage in coal-slurry
impoundment dam result in pollution of streams, rivers and ground water.
This kills fish and poisons drinking water.
Mountaintop Removal Mining (MTR):
In
this technique, the tops of coal-rich mountains are removed and the
resulting rocks are dumped into nearby valleys. The rocks bury stream
habitats altogether. This has catastrophic effect on whatever life forms
that live in or around the stream.

Marine debris
Marine
debris, also known as marine litter refers to trash in the ocean.
Though trash fouls inland waterways too, it seems to be a particular
problem in seas and oceans.
The
effects of marine debris are many. Marine animals can swallow the trash
items mistaking them with food. For example, sea turtles will eat a
plastic bag believing it to be a jelly fish. The bag cause intestinal
blockage and sometimes death. In some cases, trash can get attached to
food and consequently get ingested together with the food. This may harm
or even kill the animal that ingests it.
Discarded
or lost fishing gear (line, rope, nets) and certain trash items can get
wrapped around marine animals‟ fins or flippers and cause them to drawn
or injure their fins.
Marine
debris can also degrade coral reefs, sea grass beds, and other aquatic
habitats. This can interfere with the normal sea life.

Thermal pollution
Discharging
the hot water from a power plant into a river could affect aquatic
organisms greatly. In fact, industrial thermal pollution is a problem to
water bodies.
Aquatic
organisms are adapted to a particular temperature range. Even a small
increase in temperature can kill the organisms from thermal shock. Also
the extra heat may disrupt spawning or kill young fish.
A
high temperature warms the water and lowers the amount of oxygen that
can dissolve in that water. Insufficient dissolved oxygen forces the
aquatic organisms to increase their respiration rates. This increases
the aquatic organisms‟ susceptibility to disease, parasites and the
effects of toxic chemicals.
Global warming also causes extra heat to the oceans, leading to similar effects explained above.
Noise pollution
Noise
pollution from various ship engines and sonar systems make it difficult
for marine organisms like whales, dolphins, and porpoises to
communicate, mate, find food and avoid hazards. Excessive noise
pollution may cause damage to marine animals‟ sound-sensitive organs.
This can result to internal bleeding and even death.
Ways of Preventing Water Pollution
We
have seen that water pollution poses a great threat to aquatic
organisms, humans and the environment. Therefore, something must be done
to prevent and control this from happening.
The following are some of the methods that can be employed to prevent and control aquatic pollution:
Reducing nutrient and pesticide pollution
This
can be done by adopting good agricultural practices. These include
practicing organic farming (farming which does not involve use of
artificial chemicals), controlling soil erosion, and reducing or
controlling the use of fertilizers and pesticides in agricultural
production.
Treating sewage and industrial wastes
Sewage
and industrial waste must be treated in order to kill harmful
microorganisms and detoxify poisonous chemicals before being discharged
into water bodies. This will reduce the hazards that the chemicals and
microbes contained in wastes can cause to the environment in general.
Sewage treatment plants should be upgraded so that they can filter out
chemicals and toxins. Heated up water from industries should be cooled
down and detoxified before being release into water bodies or effluent
pools.

Stopping deforestation
Forests
act as a soil cover which prevents soil erosion. They also help to
absorb carbon dioxide from the air, the excess of which causes acid
rains. Cutting down trees indescrimately exposes the soil to erosion
agents. Soil erosion produces sediments which pollute water. So the
presence of trees prevents water pollution through cutting off
deposition of sediments into water and preventing the formation of acid
rain.
Controlling coastal development
Infrastructures
such as buildings and industries destroy the natural shorelines which
serve many purposes like fish nurseries, absorption of hurricane
impacts, and filtration of the river water entering the estuary.
Furthermore,
most of the waste from these establishments is directed into the nearby
water body. So prevention of coastal establishment will prevent this
kind of water pollution from taking place.
The
government should make and enforce laws that prohibit the establishment
of settlements and industries near water bodies. This will help control
water pollution and its impacts to the environment.
Reducing pollution from oil spills
Listed below are some of the ways that can be used to control the occurrence and repercussions of oil spills:
- Enforcing the regulations and rules that govern maintenance and inspection of commercial ships and other marine vessels that leak oil and fuel into the water.
- Cleaning oil spills as promptly as they occur.
- Converting oil tankers into double-hull ships. A double-hull ship has two complete layers of watertight hull surface. The178outer layer forms the normal hull of the ship. A second inner hull forms a protective barrier to sea water in case the outer hull is damaged and leaks.
- Educating the public how to keep oil out of the environment.
Controlling production of greenhouse gases
Greenhouse
gases, particularly carbon dioxide and sulphur dioxide, contribute to
water pollution through acid rain (acidification) and an increase in
ocean temperature (thermal pollution). This can be reduced by a number
of ways which include forestation, reducing emission of green house
gases from industries and motor vehicles, and employing good
agricultural practices.
Reducing mercury emissions
The
use of mercury in many industrial processes is being phased out.
However, some industries continue to use mercury on a large scale. Such
industries should apply the appropriate technology to prevent mercury
from being released into the environment. Where other alternatives
exist, the use of mercury should be stopped completely.
Cleaning up existing and abandoned mines
The following are some of the ways through which water pollution by mines can be controlled:
- Mining companies should clean up abandoned mines which continue to release pollutants to the environment.
- New mines should not be established in areas where they are likely to cause water pollution problems.
- Mining practices which cause water pollution should be banned.
Cleaning up chemical pollution
Chemical
pollution on land should be stopped. This is because chemicals on land
dissolve in surface run off and finally find their way into water
bodies. So preventing chemical pollution on land will automatically help
keep our water clean.
Aerial Pollution
The Concept of Aerial Pollution
Aerial pollution is the introduction of harmful substances into the earth‟s atmosphere.
Human Activities which Cause Aerial Pollution
Identify human activities which cause aerial pollution
Air
pollution can result from both human and natural actions. Natural
events that pollute the air include forest fires, volcanic eruptions,
wind erosion, pollen dispersal, and evaporation of organic compounds,
hot springs, and fumaroles. However, pollution from natural occurrences
does not occur very often.
Human activities that cause air pollution include gaseous emissions from industries, burning of fossil fuel (e.g. gas, coal), household and agricultural chemicals, and deforestation.

The following are the chief causes of air pollution:
Carbon dioxide
Carbon
dioxide (CO2) is one of the main air pollutants. The major man-made
sources of carbon dioxide are burning of fossil fuels and deforestation.
The act of deforestation removes trees that absorb carbon dioxide and
help to reduce the level of carbon dioxide gas in the atmosphere. The
natural sources of carbon dioxide include respiration, decay, volcanic
eruptions and diffusion out of the oceans.
Carbon monoxide
Carbon
monoxide (CO) is formed when fossil fuels burn in too little air
(oxygen). However, when the fuels are burnt in a plentiful supply of
oxygen, carbon dioxide (CO2) is produced. Other sources of carbon
monoxide include metal processing and chemical manufacturing activities,
forest fires, wood burning for heat or cooking and combustion of
natural gas.
Ground level ozone (smog)
This
is a product of burning fossil fuels. Smog is formed when oxides of
nitrogen (NOx) react with volatile organic compounds (VOCs) in the
presence of sunlight. It is formed in heavy traffic in hot weather, when
sunlight causes the nitrogen oxides and hydrocarbons from car exhausts
to react together.
Sunlight
and hot weather are catalysts in the NOx/VOCs reactions that cause
ground-level ozone (smog) to form. Areas with the highest concentration
of motor vehicles and industrial emissions tend to have the worst
ground-level ozone problems.

Nitrogen oxides
Nitrogen
oxides, nicknamed NOx, are highly reactive gases that contain nitrogen
and oxygen in varying molecular combinations. The oxides of nitrogen
include nitrogen monoxide (NO), nitrogen dioxide (NO2) and dinitrogen
oxide (N2O).
Nitrogen
oxides form when fuel is burned at high temperatures, such as in
automobile engines, coal-fired power plant, or any process that burns
fuel. Inside car engines and power station furnaces, the air gets so hot
that nitrogen and oxygen react together to form oxides of nitrogen.

Nitrogen
monoxide (NO) is also formed in the atmosphere by lightning where
nitrogen and oxygen combine together to form the oxide:
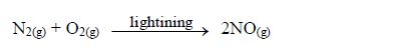
NOx
and the pollutants formed from it can be transported by windover long
distances. Thus, these types of pollution are notconfined to areas where
they are produced, and controlling them isbest done using regional and
national plans.
Sulphur compounds
Compounds
of sulphur that pollute the air include sulphur dioxide(SO2), sulphur
trioxide (SO3) and hydrogen sulphide (H2S).Sulphur dioxide is the most
important air pollutant among thecompounds of sulphur.
Coal,
oil and gas all contain sulphur and sulphur compounds asimpurities.
When these sulphur-containing fuels are burnedsulphur dioxide gas is
formed. The gas is also formed duringpetroleum refinery.
Particulate matter (PM)
Particulate
matter comprises of extremely small solid particles andliquid droplets.
These are mainly particles of carbon (soot) fromburning coal in power
stations and petrol in motor engines. Theother particles include smoke,
nitrates, sulphates, dust particles,and organic chemicals. Exhaust fumes
from leaded petrol alsocontain particles of lead.
Lead
Lead
can pollute the air. Lead in air comes mainly from industrialactivities
like lead smelting, metal processing, lead-acid batterymanufacturing,
waste incineration and power generation. Lead isalso produced from
combustion of airplane fuel. Leaded petrol isstill used in some
developing countries. Wind-blown soil and roaddust can also contain
naturally-occurring lead as well as lead fromother sources.
Chlorofluorocarbons (CFCs)
Chlorofluorocarbons
are organic compounds made up of carbon, chlorine and fluorine atoms.
CFCs are widely used as refrigerants in refrigerators and air condition
systems, solvents in cleaners, blowing agents in the production of foam
propellants in aerosol cans, and as fire extinguisher chemicals.
Hazards Caused by Aerial Pollution
The following are some effects of air pollution.
Health problems
Exposure
to air pollutants can cause serious health problems. The level of
effects usually depends on the length of time of exposure as well as the
kind and concentration of the pollutants.
Short
term air pollution can aggravate or complicate medical conditions of
individuals with asthma, emphysema, bronchitis, lung and heart diseases,
and respiratory allergies. Long-term health effects can include chronic
respiratory diseases, lung cancer, heart diseases, and even damage to
the brain, nerves, liver or kidneys.
Inhaling or ingesting lead can damage the brain and peripheral nerves resulting to retardation, behaviour disorders and memory loss. It can also cause high blood pressure, heart diseases, anaemia and reproductive disorders.
Carbon
monoxide is poisonous even in small concentrations. It combines with
haemoglobin in the blood and stops it from transporting oxygen to the
body cells. This can cause harmful health effects by reducing oxygen
delivery to the body tissues and vital organs (most notably the heart
and the brain). Inhaling high levels of carbon monoxide can cause vision
problems, physical or mental impairment, and even death.
Global warming
Global
warming is the increase in the average temperature of the earth‟s
atmosphere as a result of the greenhouse effect. Greenhouse gases in the
atmosphere allow ultraviolet radiations to pass through them and reach
the earth. As the earth‟s surface gets heated up, some of the heat is
radiated back to the atmosphere. A layer of the greenhouse gases in the
atmosphere, acting as a blanket, prevents the heat from escaping to the
upper atmosphere. This causes excess heat in the air around the earth
(atmosphere), a phenomenon called global warming.
Global warming is mainly caused by human activities such as fossil fuel burning and deforestation, which produce green house gases such as carbon dioxide and some oxides of nitrogen (NOx). Carbon dioxide is one of the greenhouse gases that contribute to global warning. Indeed, carbon dioxide is responsible for over 50% of the global warming.
One
member of the NOx family, nitrogen oxide (N2O), is also a potent
greenhouse gas. The gas contributes to the greenhouse effect.
Global warming may result in melting of the polar ice caps, causing an increase in levels of the ocean water. This may lead to flooding of the coastlands and islands. Other effects include increase in the average temperature of the earth, change in weather patterns, and increased desertification, which would in turn cause reduction of the arable land.
Depletion of the ozone layer
The
production and emission of chlorofluorocarbons (CFCs), is the leading
cause of ozone layer depletion. CFCs accounts for almost 80% of the
total depletion of ozone.Ozone is a triatomic form of oxygen (O3), found
in the earth's atmosphere. When the CFCs reach the stratosphere (a
layer of the atmosphere with high concentrations of ozone), they are
broken down by the intense sun rays to release chlorine radicals (•Cl).
These radicals then react with the ozone molecules splitting them into
oxygen atoms and free oxygen molecules. This is how the ozone layer is
getting depleted.
Acid rain
Human
activities produce harmful gases such as oxides of nitrogen and sulphur
which are released into the atmosphere. These gases are responsible for
the formation of acid rain.
Sulphur
dioxide gas in air dissolves in rain water to form acid rain and other
sulphur compounds. Acid rain is formed when the sulphur dioxide
dissolves in water vapour from the clouds and combines with oxygen from
the atmosphere to form an acid rain.
SO2(g) + H2O(l) + ½O2(g) H2SO4(aq)
Sulphur dioxide attacks the lungs, and can affect people with asthma and heart diseases very badly.
Nitrogen
monoxide and nitrogen dioxide also play a great part in the formation
of acid rain and photochemical smog. Acid rain is formed by the reaction
of NOx, oxygen of the air and water vapour. 4NO2(g) + 2H2O(l) +
O2(g)→4HNO3(aq)
These
acidic solutions then fall on the ground in the form of acid rain. Acid
rain damages plants and corrodes many ancient buildings, monuments and
sculptures made of marble. It greatly affects the aquatic life due to
the acidification of lakes and streams.
Eutrophication
Even
though most of the nutrient pollution comes from fertilizers and
animals wastes that run off from farm fields, deposition of nitrogen
from fossil fuel air pollution adds significantly to the problem.
Eutrophication
causes algal bloom which prevents the penetration of air and light into
a water body. This deprives aquatic life of oxygen and light. When
algae die, bacteria act on them. The bacteria use dissolved oxygen in
the water, depleting the gas from the water. The depletion of oxygen in
the water (hypoxia) causes a reduction and even death of certain fish
and other aquatic organisms.

Effect on wildlife
Just
like humans, animals also face some devastating effects of air
pollution. Toxic chemicals present in air can force wildlife species to
move to new places and change their habitat. The toxic pollutants
deposited in the water can also affect sea animals.
Reduced visibility (haze)
Smog reduces visibility, making activities such as driving, surveying and flying difficult.
Different Methods of Preventing Air Pollution
The following are some remedial measures that can be taken to control air pollution:
Emphasis on clean energy sources
Clean
energy sources are those which do not produce harmful substances during
production and use. They include wind energy, solar energy, hydro
electric power, and geothermal energy.
Installation
of solar panels for home will help curb air pollution. This will help
reduce dependency on fossil fuels, wood and charcoal which produce and
emit harmful substance into the atmosphere during use. Also the use of
wind energy, hydro electricity and geothermal sources of energy will
greatly reduce the generation of air pollutants.

Using energy-efficient devices
Use
devices that consume less electricity not only help lower electricity
bills but also reduce the pollution. The domestic appliances which serve
energy include energy-serving bulbs, which can be purchased from local
shops.

Using public transport
Use
of public mode of transport will reduce the number of motor vehicles on
the road, and consequently, the emission of harmful gases by car
exhaust system.

Reducing pollution from fossil fuels
Several steps are being taken to help cut down pollution from fossil fuel, for example:
- the exhaust of new cars are fitted with catalytic converters in which harmful gases are converted to harmless ones;
- manufacturers are looking at ways to make car engines more efficient so that they can use less petrol, and other alternative fuels;
- coal is turned into smokeless fuel for use in homes; and
- scientists are looking at ways to make homes and factories more energy efficient, so that we can burn less fuel, not more.
Use of air pollution control devices
Air
pollution control devices should be installed in industrial plants and
any other places where air pollutants are produced in tremendous
quantities.Most plants include areas for treating effluent (waste
liquid) and waste gases before they are released, for example: limestone:
This will neutralize acidic liquids, or the sulphur dioxide in waste
gases from burning coal. Gases leaving the power station are passed
through limestone, which reacts with the sulphur dioxide to form calcium
sulphate. Calcium sulphate is used to make plaster for building
industry.
charcoal:
This is porous and has a large surface area. It is excellent at
absorbing impurities. So it is used for filtering both air and liquids.
scrubbers:
The waste gas is sprayed with water to dissolve harmful compound before
they reach the chimneys. incinerators: Harmful waste gases such as
solvent fumes are burned in an incinerator to give harmless products.
ion
exchangers: when waste liquid flows through an ion exchanger, harmful
ions (such as mercury or cadmium ions) are replaced by harmless ones.
Ion exchangers can be designed to remove any ions you wish.
electrostatic
precipitators: In these, an electric current is passed through smoke on
its way to the chimneys. The dust particles get charged and cling to
electrodes. This method is used at cement factories to stop cement dust
escaping from kilns. The trapped dust is returned to the cement store.
Law enforcement
Environmental
pollution laws which ensure safe disposal of wastes should be enacted
and implemented. Policies should be put in place to prohibit any kind of
pollution by vehicles and industries. Vehicles which emit a lot of
exhaust gases should not be allowed to operate. The owners of a plant
which pollutes the environment should be fined or the plant should even
be closed down.
The National Environment Management Council (NEMC) has put in place laws and regulations that control careless pollution of the environment. Violators of the environment sanitation laws are fined, sentenced and even stopped from operating.
Safety Measures to Protect Industrial Workers from Gaseous Pollution
People
working in industries face serious health risks from harmful gases and
chemicals used in industrial processes. These gaseous pollutants cause
various health problems to workers, including illness and disorders upon
contacting the skin or getting inhaled into the body.
Harmful
gases get into the worker‟s bodies through breathing contaminated air,
eating food contaminated with gaseous fumes or via skin contact.
Safety measures should be ensured in order to protect the workers from being affected by the hazardous fumes or other chemicals. The following are some safety measures that should be taken to protect industrial workers from gaseous pollution.
Eliminate or substitute hazardous chemicals
The
most effective way of controlling hazards caused by chemicals is to
stop using or substitute the hazardous chemicals with the harmless ones.
Many industries are now using harmless chemicals in place of the usual
hazardous chemicals in industrial processes and operations so as to
safeguard the heath of workers.
Examples of substituting for toxic substances are:
- using water-based paints or glues instead of those that are organic solvent based (figure 4.17);
- using water-detergent solutions instead of solvents; and
- using trichloromethane as a degreasing agent instead of trichloroethylene.
Shield processes that release harmful substances
The
processes that involve the use of hazardous chemicals or release
harmful products should be performed in chambers. This will prevent the
harmful substances from being released into the environment.
Use of protective gears
Workers
should be provided with the necessary protective equipment and be
trained on the correct use of each gear. Each worker should put on
protective equipment to ensure personal safety at work place. The
protective equipment include safety glasses, goggles, earmuffs and
plugs, gloves, safety shoes, helmets, aprons, overalls and respirator
(which covers the mouth and nose of the worker to prevent the entry of
chemicals into the body by inhalation).If possible, workers must take
showers at workplace before leaving work to avoid bringing chemicals at
home. Dirty clothes should be left at work. If they must be washed at
home, washing should be done separately, never with the family wash!

Ventilation
Ventilation
means trapping the contaminants (fumes, gases, vapours or mists)
released into the air from the process or operation and preventing them
from entering the breathing zone of the workers. The trapped
contaminants are conveyed by ducts to a collector (cyclone, filter
house, scrubbers or electrostatic precipitators) where they are removed
before the air is discharged into the outside environment. This is
accomplished by a special exhaust system or by increasing the general
ventilation.
Environmental Conservation
The Meaning of Environmental Conservation
Environmental
conservation is the protection and preservation of natural resources
from destruction, wastage or loss. Thus, conservation of the environment
involves the conservation of the natural resources. The natural
resources include soil, minerals, water, air, animals and plants.
Importance of conserving the environment
We
depend on the environment for our livelihood. It is, therefore,
important to conserve the environment for our survival and sustainable
development. The following are some of the reasons why environmental
conservation is crucial.
Sustenance of life
The
environment contains all the resources that sustain human life. These
include soil, water, air, animal and plants. Environmental conservation
ensures the protection of these resources for the continued sustenance
of life on earth.
Protection of animal and plant species
Plants
and animals maintain the ecological balance in the ecosystem. Man has
been exploiting these resources for quite a long time, to the extent
that some plant and animals species are in danger of disappearing from
the earth‟s surface. Human activities which lead to destruction of
wildlife include hunting, agriculture, settlement, mining and trade.
Environmental conservation aims at protecting plants and animals from
destruction.

Economic value
Economic
activities such as fishing, agriculture, mining and navigation depend
on the natural resources. For sustainable economic development, these
resources need to be conserved so as make them available in sufficient
amounts.
Wildlife
conservation makes game and plant resources available for attracting
tourists. Tourism earns the country foreign exchange. It also creates
employment opportunities to local people who are employed to conserve
wildlife resources.
Future generations
Environmental conservation aims at preserving the resources so as to make them available for the upcoming generations.
Beauty/austhecy
The
natural resources give the environment beautiful scenery for example,
flower are attractive to look at. Forests provide shade and the air
around forest is also cold. Thus, environmental conservation initiative
helps to preserve this natural beauty.

Right Attitudes, Values,and Behaviours towards Environmental Conservation
The following are some of the measures taken to conserve the environment.
Setting up organizations and institutions
Many
organizations have been set up for the purpose of conserving the
environment. These organizations include international organizations,
government ministries and agencies, and non-government
organizations.Examples of government ministries and agencies include the
Ministry of Natural Resource and Tourism and the National Environment
Management Council (NEMC) respectively. An example of international
agencies concerned with environmental conservation issues is the United
Nations Environment Programme (UNEP).
Legislation
The
laws that govern environmental conservational are being made and
enacted. The laws aim at curbing environmental destruction by punishing
or fining those individuals who destroy our environment.
Education
People
are being educated about the importance of conserving the environment
for their benefits. Environment education is being continually offered
in schools, colleges, universities and other organizations. Academic
institutions now offer courses such as environmental studies,
environmental management, and environmental engineering that teach the
history and methods of environmental protection.
Research
Research
is being carried out on the best ways to protect and conserve the
natural resources. Some of the areas of research include
- alternative sources of energy;
- methods of preventing and controlling pollution;
- sustainable use of natural resources;
- recycling and reuse of material;
- environmental impact assessment.
Pollution prevention and control
Much
effort is being directed towards prevention and control of pollution.
This is being done by setting up recycling factories, rehabilitating
polluted areas and enacting laws and policies to control pollution,
among others.
International agreement
International
organizations such as the United Nations (UN) and regional blocks such
as the European Union (EU) have drafted agreements which provide
guidelines on the conservation of the environment. Member nations commit
themselves by signing and implementing these agreements. Most of the
agreements are legally binding for countries that have formally ratified
them. Such agreements include the Kyoto Protocol which was agreed upon
in Kyoto, Japan on December 11, 1997.
Personal involvement
It
is crucial that everyone gets involved in the conservation of the
environment at individual level. Each citizen should feel obliged to
take part in conserving the environment. We cannot let the task be done
by the government, agencies and the international organizations alone.
This is because we are all involved in environmental destruction, and
the effect of this destruction affects all of us.
The following are some of the ways in which you can participate in environmental conservation:
- Plant more trees at home and farm fields, school and village forest. Do not cut down tress indiscrimately because doing so leaves the soil bare and vulnerable to soil erosion.
- Always dump litter in areas designated for waste disposal and in litter bins. Do not just throw dirt anywhere and carelessly.
- Do not start fires near forests. Farmers should not prepare their farm fields by burning the vegetation because the fire can spread and destroy trees and nearby forests. Fire also kills important soil microorganisms, thus curtailing soil fertility and productivity.
- Do not harm domestic and wild animals by any means. Be kind to animals and treat them humbly.
- Convey environmental conservation education to all people. Let them know the importance of conserving and living in a clean environment.
- Participate in environmental conservation programmes and tasks. These include World environment Day (June 5, every year) and clear-up exercises in the local area or town.
Global Warming
Global
warming refers to an equivocal and continuing rise in the average
temperature of the air and sea at the earth‟s surface. Since the early
20th century, the global air and sea surface temperature has increased
by about 0.8°C, with about two-thirds of the increase occurring since
1980. Each of the last three decades has been successively warmer at the
earth‟s surface than preceding decades since 1850.
The
recent rapid warming was caused by human activities which contribute to
the production of greenhouse gases, such as carbon dioxide, that trap
heat in the earth‟s atmosphere. It is predicted that the continuation of
these activities will result in 1.8–4°C average temperature increase
over the next century.
The Global Warming in Terms of ‘Green House’ Effect
The
greenhouse effect is a phenomenon in which the greenhouse gases in the
earth‟s atmosphere form a layer that shields the heat emitted by the
earth and prevents it from escaping out into space.
The
sun‟s light (also called solar radiation) passes through the atmosphere
and hits the earth‟s surface. Energy from the sunlight is absorbed by
the earth‟s surface in form of heat, making it warmer. Some of this heat
energy (also called infrared radiation) is released back into the
atmosphere. Certain gases, called greenhouse gases, form a layer in the
earth‟s upper atmosphere that prevents much of this heat from leaving
the atmosphere and going out into space. These gases act like the glass
of a greenhouse or window: they let light in, but keep some of the heat
from passing back out.
How the Major “Greenhouse” Gases are Produced
Scientific
understanding of the cause of global warming has been increasing.
Global warming is mostly caused by increasing concentrations of
greenhouse gases in the atmosphere. The greenhouse gases include water
vapour (H2O), carbon dioxide (CO2), methane (CH4), dinitrogen oxide or
nitrous oxide (N2O), ozone (O3) and chlorofluorocarbons (CFCs). The
following greenhouse gases are the main contributors to global warming.
They are the main causes of global warming.
Carbon dioxide
Carbon
dioxide is the main greenhouse gas. The gas contributes over 50% of the
greenhouse effect. It is because of this reasons that man is struggling
to reduce carbon dioxide emissions. The following are some of the
man-made sources of carbon dioxide in the atmosphere.
Deforestation
Green
plants absorb carbon dioxide gas from the atmosphere and use it to
manufacture their food through the process of photosynthesis. Cutting
down trees means that a few trees are left to absorb carbon dioxide gas
from the air. This has led to the increase in the amount of carbon
dioxide in the atmosphere.

Combustion of fuel
Burning
of fossil fuel such as wood, coal, petroleum and natural gas, releases
carbon dioxide into the atmosphere. The gas is produced during
combustion of these fuels in car engines, power stations, industries,
etc.
Methane
The
main source of methane is from agricultural activities. It is released
from wetlands such as rice fields and from animals, particularly
cud-chewing animals, like cattle. The emission of methane gas into the
atmosphere, therefore, increases with increase in agricultural
activities. Since 1960s the amount of methane in the air has increased
by 1% per year, twice as fast as the build-up of carbon dioxide.
Methane is also produced by the decomposition of waste materials by bacteria. It is the major component of natural gas. The gas is also produced during the mining of coal and oil (as natural gas) and when vegetation is burnt.
Nitrous oxide (dinitrogen oxide, N2O)
Dinitrogen
oxide is produced from both man-made and natural processes. Human
activities which produce dinitrogen oxide include combustion of fossil
fuels in vehicles and power stations, use of nitrogenous fertilizers and
burning of vegetation and animal waste. During combustion of fuel in
automobile engines, the air gets so hot that nitrogen reacts with oxygen
to form dinitrogen oxide.
The
gas is also produced by digesting bacteria, and is part of the nitrogen
cycle, one of the most important natural processes on earth.
Chlorofluorocarbons (CFCs)
The
sources of CFCs in the atmosphere include refrigerators, air
conditioners and aerosols. CFCs are extremely effective greenhouse
gases. One CFC molecule is about 10,000 times more effective in trapping
heat than a carbon dioxide molecule. Some of them are up to 14,000
times effective than carbon dioxide, the main greenhouse gas.
Climatic Conditions caused by Global Warming
Global
warming is expected to have far-reaching, long-lasting and, in many
cases, devastating consequences for planet earth. The following are some
effects of global warming:
Increase in average temperatures
One
of the most immediate and obvious impacts of global warming is the
increase in temperatures on the world. The average global temperature
has increased by about 0.8°C over the past 100 years. Scientists predict
that the earth‟s average temperature will increase by between 1.4 and
5.8°C by the year 2100.Increase in global temperature will affect both
the land and the ocean environments. The average temperature of the
oceans has increased significantly in the past few decades, causing
negative effects on marine life.
When the ocean water gets warm, the algae in the ocean tends to produce toxic oxygen compounds called superoxides which are damaging for the corals. Global warming is threatening the coral reefs to a great extent, and the fact is that if coral reefs are wiped off the planet, it will affect one third of planet‟s marine biodiversity, as well as other ecosystems related to the coral reefs directly or indirectly.
Extreme weather events
Extreme
weather events include record-breaking high or low temperatures, floods
or intense storms, droughts, heat waves, hurricanes and tornadoes, etc.
These are effective measures of climate change and global warming.

Scientists
project that extreme weather events, such as heat waves, droughts,
blizzards and rainstorms will continue to occur more often and with
greater intensity due to global warming. Other effects of extreme
weather events include:
- higher or lower agricultural yields;
- melting of arctic ice and snowcaps. This causes landslides, flash floods and glacial lake overflow;
- extinction of some animal and plant species; and
- increase in the range of disease vectors, that is, organisms that cause diseases.
Change in world’s climate patterns
It
is forecasted that global warming will cause climate patterns worldwide
to experience significant changes. Climate change resulting from
increasing temperatures will likely include changes in wind patterns,
annual precipitation and seasonal temperature variations.
Climatic
patterns in most parts of the world have already changed. Rains fall
when least expected and at irregular intervals. This has greatly
affected the timing of planting and harvesting activities. Sometimes the
rains fall so heavily to cause floods, or too little leading to
drought.
Most
of the arable land that once used to be productive is slowly turning
arid. With time, farmers will run short of the land for cultivation, a
fact that will result in famine.
Because
high levels of greenhouse gases in the atmosphere are likely to remain
high for many years, these changes are expected to last for several
decades or longer.
Rise in sea levels
Continued
increase in the global temperature will cause the melting of ice caps
in the poles and mountain glaciers. Melting polar ice and glaciers are
expected to raise sea levels significantly. Global sea levels have risen
about 8 inches since 1870 and the rate of increase is expected to
accelerate in the coming years. If current trends continue, many coastal
areas will eventually be flooded.
Scientists
predict that by the year 2100 the sea level will raise by at least 25m,
leading to coastal flooding that will displace millions of people.
Small islands in the Caribbean, South Pacific, Mediterranean and Indian
Ocean will be totally covered by ocean waters.
Ocean acidification
As
levels of atmospheric carbon dioxide increase, the oceans absorb some
of it. This increases the acidity of seawater. Since the Industrial
Revolution began in the early 1700s, the acidity of the oceans has
increased about 25%.
Because
acids dissolve calcium carbonate, seawater that is more acidic has a
drastic effect on organisms with shells made of calcium carbonate, such
as corals, mollusks, shellfish and plankton. The acid water is likely to
dissolve the carbonaceous shells, thus endangering the lives of these
sea creatures. Change in ocean acidity will also affect fish and other
aquatic animals and plants.
If
current ocean acidification trends continue, coral reefs are expected
to become increasingly rare in areas where they are now common.
Effects on plants and animals
The
effects of global warming on the earth's ecosystems are expected to be
profound and widespread. Many species of plants and animals are already
moving their range northward or to higher altitudes as a result of
warming temperatures.
Additionally, migratory birds and insects are now arriving in their summer feeding and nesting grounds several days or weeks earlier than they did in the 20th century.
Warmer
temperatures will also expand the range of many disease-causing
pathogens that were once confined to tropical and subtropical areas,
killing off plant and animal species that formerly were protected from
disease.
These
and other impacts of global warming, if left unchecked, will likely
contribute to the disappearance of up to one-half of the earth's plants
and one-third of animals from their current range by 2050.
Effects on humans
As
dramatic as the effects of climate change are expected to be on the
natural world, the projected changes to human society may be even more
devastating.
Agricultural
systems will likely be affected badly. Though growing seasons in some
areas will expand, the combined impacts of drought, severe weather, lack
of snowmelt, greater number and diversity of pests, lower groundwater
tables and a loss of arable land could cause severe crop failures and
livestock shortages worldwide.
This
loss of food security might, in turn, create havoc in international
food markets and could spark famines, food riots, political instability
and civil unrest worldwide.
The
effect of global warming on human health is also expected to be
serious. An increase in mosquito-borne diseases like malaria and dengue
fever, as well as a rise in cases of chronic conditions like asthma, are
already occurring, most likely as a direct result of global warming.
Ways of Preventing Global Warming
The
effects of greenhouse gases in the atmosphere will continue to be felt
for many years. This is because greenhouse gases remain in the
atmosphere for a very long period of time. For example, carbon dioxide
molecules can remain in the atmosphere for a period ranging between 50
and 100 years while that of a CFC molecule is approximately 110 years.
This means that global warming will continue even if the emission of
greenhouse gases is reduced to very low levels.
A
growing number of business leaders, government officials and private
citizens are increasingly concerned about global warming and its
implications. Thus they are proposing steps to reverse the trend. The
following are some steps that can be taken to reduce the emission of
greenhouse gases into the atmosphere:
- Conserving the energy so as to reduce the use of fossil fuels which produce greenhouse gases. Such measures can be taken by using public transport to reduce the number of motor vehicles on the road and using cars that consume a little fuel.
- Minimize the use of deodorants, as they contain CFCs (chlorofluorocarbons) that contributes to the ozone depletion, which in turn gives rise to most destructive effects.
- Planting more trees (afforestation) and avoiding cutting down trees (deforestation) carelessly. This is because forests play an important role in absorbing carbon dioxide, thus reducing the amount of carbon dioxide in the atmosphere.
- Encourage the use of renewable sources of energy like wind, biomass, solar, and geothermal energy. The use of solar power and biomass should be installed widely. But there are a few obstacles that are delaying the use of these technologies.
- Raise awareness! Educate people about global warming and its disastrous effects. Share various solutions to stop global warming. Make sure you take initiatives to help conserve the environment and encourage others to do the same.
- Countries, including Tanzania, have ratified the international agreements aiming at minimizing the emission of greenhouse gases. One of those agreements is the Kyoto Protocol.
Ozone Layer Destruction
The Meaning of Ozone Layer and its Importance of to Life on Earth
The
atmosphere is divided into five layers. From the closest and thickest
to the farthest and thinnest, the layers are: troposphere, stratosphere,
mesosphere, thermosphere and exosphere.
The
majority of the atmosphere‟s ozone is in the stratosphere, which
extends from 10 kilometres to 50 kilometres above the earth‟s surface.
The ozone layer is a layer of gaseous ozone (O3) in the stratospheric atmosphere. The earth‟s stratospheric ozone plays an important role in absorbing ultraviolet radiations. Ultraviolet radiations (UVR), are high energy electromagnetic waves emitted from the sun. UV radiations include UV-A, the least dangerous form of UV radiations, UV-B, and UV-C, which is the most dangerous. UV-C is unable to reach the earth's surface due to stratospheric ozone's great ability to absorb it. The real threat comes from UV-B, which can enter the earth's atmosphere, and has adverse effects.
UV-B
radiation (the higher-energy UV) causes skin cancer, eye cataracts, and
can lead to genetic damage.Although natural phenomena can cause
temporary ozone loss, chlorine and bromine released from man-made
compounds such as CFCs are the main cause of ozone layer depletion
(destruction). They are also greenhouse gases and contribute to global
warning.
CFCs
are made up of chlorine, fluorine and carbon atoms and are more
extremely stable. This extreme stability allows the CFCs to slowly make
their way into the stratosphere. They can remain in the atmosphere for
20 to 120 years or more.
In
the stratosphere, CFCs are broken down by UV rays from the sun,
releasing free chlorine radicals (•Cl). Chlorine atom (radical) reacts
with an ozone molecule (O3) to form chlorine monoxide, ClO, and one
molecule of oxygen (O2). Then chlorine monoxide reacts with a second
molecule of ozone to yield the original chlorine atom and two molecules
of oxygen.
- 1. CCl3F → •CCl2F + •Cl2.
- •Cl + O3 → •ClO + O23.
- •ClO + O3 → •Cl + 2O2
After
each reaction, the freed chlorine atom (radical) is able to begin
destructive cycle again with another ozone molecule. It is estimated
that one chlorine radical can destroy up to 100,000 molecules of ozone.
Chemical Substances which Destroy the Ozone Layer
The
main ODS are chlorofluorocarbons (CFCs), hydrochlorofluorcarbons
(HCFCs), carbon tetrachloride and methyl chloroform. Halons (brominated
fluorocarbons) also play a big role. Their application is quite limited:
they're used in specialized fire extinguishers. But the problem with
halons is they can destroy up to 10 times as much ozone as CFCs can.
Hydrofluorocarbons
(HFCs) are being developed to replace CFCs and HCFCs, for uses such as
vehicle air conditioning. HFCs do not deplete ozone, but they are strong
greenhouse gases. CFCs are even more powerful contributors to global
climate change, though, so HFCs are still the better option until even
safer substitutes are discovered. The following are the main
ozone-depleting substances:
Chlorofluorocarbons (CFCs)
- The most widely used ODS, accounting for over 80% of total stratospheric ozone depletion.
- Used as coolants in refrigerators, freezers and air conditioners in buildings and cars manufactured before 1995.
- Found in industrial solvents, dry-cleaning agents and hospital sterilizers.
- Also used in foam products such as soft-foam padding (e.g. cushions and mattresses) and rigid foam (e.g. home insulation)
Halons
- Used in some fire extinguishers, in cases where materials and equipment would be destroyed by water or other fire extinguisher chemicals.
Trichloroethane (CH3CCl3)
- Used mainly in industry as a solvent in many products and for metal cleaning.
Carbon tetrachloride (CCl4)
- Used in solvents and some fire extinguishers.
Hydrochlorofluorocarbons (HCFCs)
- HCFCs have become major, “transitional” substitutes for CFCs. They are much less harmful to stratospheric ozone than CFCs are. However, HCFCs still cause some ozone destruction and are potent greenhouse gases.
Possible effects of ozone depletion
As
ozone depletes in the stratosphere, it forms a 'hole' in the layer.
This hole enables harmful ultraviolet rays to enter the earth's
atmosphere. Ultraviolet rays of the sun are associated with a number of
health-related and environmental issues. Let us take a look at how ozone
depletion affects different life forms.
Impact on humans
Skin
cancer: Exposure to ultraviolet rays poses an increased risk of
developing several types of skin cancers, including malignant melanoma,
basal and squamous cell carcinoma.
Eye damage: Direct exposure to UV radiations can result in photokeratitis (snow blindness), and cataracts.
Immune system damage: Increased exposure to UV rays may lead to impairment of the immune system.
Accelerated aging of skin: Constant exposure to UV radiation can cause photo allergy, which results in the outbreak of rash in thin-skinned people.
Other effects: Ozone chemicals can cause difficulty in breathing, chest pain, throat irritation, and can hamper lung functioning.
Effects on amphibians
Ozone
depletion is listed as one of the causes of the declining numbers of
amphibian species. Ozone depletion affects many species of amphibians at
every stage of their life cycle. Some of the effects are mentioned
below:
- Hinders growth and development in larvae.
- Changes behaviour and habits.
- Causes deformities in some species.
- Decreases immunity: Some species have become more vulnerable to diseases and death.
- Retinal damage and blindness in some species.
Effects on marine ecosystems
In
particular, plankton (phytoplankton and bacterioplankton) are
threatened by increased UV radiation. Marine phytoplankton play a
fundamental role in both the food chain as well as the oceanic carbon
cycle. Plankton play an important role in converting atmospheric carbon
dioxide into oxygen. Ultraviolet rays can influence the survival rates
of these microscopic organisms, by affecting their orientation and
mobility. This eventually disturbs and affects the entire ecosystem.
Impact on plants
In
some species of plants, UV radiation can alter the time of flowering,
as well as the number of flowers. Plant growth can be directly affected
by UV-B radiation. Despite mechanisms to reduce or repair these effects,
physiological and developmental processes of plants are affected.
Methods of Protecting the Ozone Layer
Suggest methods of protecting the ozone layer
The
most effective way of controlling the depletion of ozone layer is to
ban the production and use of ozone depleting substances (ODS). The most
dangerous of these substances are chlorofluorocarbons (CFCs). Since
their introduction, CFCs have been used as:
- refrigerants in refrigerator and air conditioning units;
- propellants in aerosol cans;
- solvents and blowing agents for insulation foams;
- cleaners in electronic industry;
- fire extinguisher chemicals.




