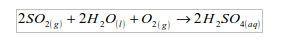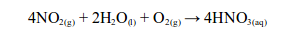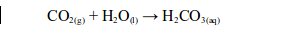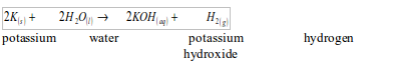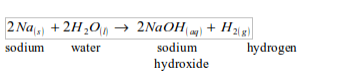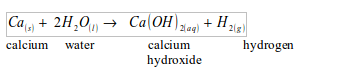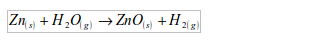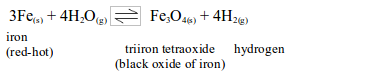WATER
Occurrence and Nature of Water
Uses of Water
Uses of Water
Water
is one of the most vital natural resources for all life on earth. The
availability and quantity of water have always played an important part
in determining not only where people can live, but also their quality of
life. Even though there always has been plenty of fresh water on earth,
water has not always been available when and where it is needed, nor is
it always suitable for all uses. Water must be considered as a finite
resource that has limits and boundaries to its availability and
sustainability for use.
Where
water supply is limited, conflicts may result between and among the
various uses. The balance between supply and demand for water is a
delicate one. The availability of usable water has and will continue to
dictate where and to what extent development will occur. Water must be
in sufficient supply for an area to develop, and an area cannot continue
to develop if water demand far exceeds supply.
Water has numerous uses in life. The following are some of the uses of water:
- Biological use: Water is essential to life. Most of the reactions in animals and plants take place in solutions in water. Plants absorb minerals from the soil in solution form. Animals and plants are found near or in areas where water can be found.
- Domestic use: Domestic water use is probably the most important daily use of water for most people. It includes water that is used in the home every day including water for normal household purposes such as washing clothes and dishes, drinking, bathing, food preparation, flushing toilets, and watering lawns and gardens, etc.
- Industrial use: Water is a valuable resource to the nation’s industries for such purposes as processing, cleaning, transportation, dilution, and cooling in manufacturing industries. Major water-using industries include cloth, steel, chemical, paper, and petroleum refining. Industries often reuse the same water repeatedly for more than one purpose. Water is used as a solvent in many industrial processes. It is also used for cooling certain parts of machines.
- Irrigation: Water is artificially applied to farm, orchard pasture, and horticultural crops, as well as leaching of salts from the crop root zone in sodic soils. Non-agricultural activities include self-supplied water to irrigate public and private flower gardens, loans, football pitches, etc. Crop production in areas that receive little rainfall per year can be achieved through the practice of irrigation. Water for irrigation purposes can be drawn from rivers, lakes, swamps and even from seas.
- Water as a solvent: Water is regarded as a universal solvent. It dissolves almost all substances. For this reason, it is used for dissolution of chemicals ranging from poisonous chemicals used in agriculture to non-poisonous chemicals used in hospitals, laboratories, research stations and for other general purposes.
- Cooling and heating: Due to its high specific heat capacity, water is used as a coolant for cooling automobile engines and other machines. Hot water is used during winter for heating homes in temperate countries. In higher plants, evaporation causes a cooling effect and therefore helps to cool plant organs. During hot weather, some animals tend to wallow in water in order to cool their bodies either through evaporation or by water itself.
- Habitat: Water is a habitat for fish and all aquatic animals and plants.
- Livestock use: This includes water for stock animals, feedlots, dairies, fish farms and other non-farm animals. In arid regions of Tanzania, the Government has constructed dams to supply water to cattle, and for some domestic uses.
- Mining: Water is used in mines for extraction of naturally occurring minerals: solids, such as coal and ores; liquids, such as crude petroleum; and gases, such as natural gas. This includes quarrying, milling (such as crushing, screening, washing, and flotation), and other operations as part of mining activity.
- Generation of electricity: Hydroelectric power is generated by river water. Fast-moving river water (especially in waterfalls and cataracts) is used to turn turbines to generate hydroelectricity that is supplied to homes, industries, towns, etc. Most of the electricity we use at home is generated by this means. Only a small portion is generated through other means.
- Navigation and recreation: People, goods and services can be transported via water bodies like rivers, lakes and oceans by using vessels such as boats, dhows, canoes and ships. Water is also used for sports such as swimming, canoeing, fishing, yachting, water skiing, and many other sports carried out on, in and under the water.
The Solubility of Different Substances in Water and Organic Solvents
Water
is a very good solvent for many ionic substances. There are few
substances, which do not dissolve in water to some extent. Even when you
drink a glass of water, you are also drinking a little of the glass as
well. The amount is very small indeed, but for certain experiments
ordinary glass vessels cannot be used as containers for water because of
this solvent effect. Water is the commonest solvent in use, but other
liquids, are also important. The other solvents are generally organic
liquids such as ethanol, propanone, trichloroethane, etc. These organic
solvents are also important because they will often dissolve substances
that do not dissolve in water. The following table shows an example of
substances that dissolve in water.
Substances soluble and insoluble in water
| Soluble compounds | Insoluble compounds |
| 1 All common sodium, potassium and ammonium salts | |
| 2. All common nitrates of metals | |
| 3. All common chlorides except………………….. | silver, mercury (I) and lead chloride |
| 4. All common sulphates except………………….. | lead, barium and calcium sulphates |
| 5. Sodium, potassium, and ammonium carbonates… | but other common carbonates are insoluble |
| 6. Sodium, potassium and ammonium hydroxides… | but other common hydroxides are insoluble. |
When salt is added to water and the mixture stirred, the salt dissolves. The product formed is termed as a solution. The solid that dissolves is known as a solute and the liquid (water) in which a solute dissolves is a solvent.
We can continue to add more salt and stir until no more salt dissolves. At this point, the water has dissolved the maximum amount of salt possible. The amount of salt dissolved denotes the maximum amount of salt which can normally be held in solution.
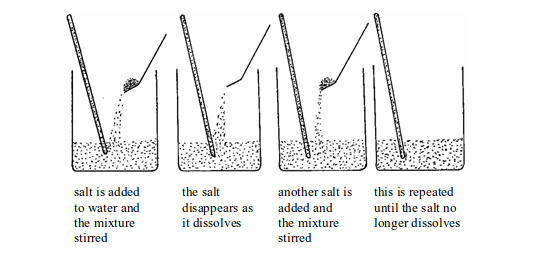
Adding a salt to water
The solution made is called saturated solution. The amount of the salt that has dissolved is called the solubility of the salt in water. The solubility
of a substance is usually expressed as the mass of the substance
dissolved in 100g of water. Solubility is sometimes expressed in moles
of solute per dm3 of solution at that temperature.
To give a quantitative meaning to solubility, it is necessary to fix the amount of the solvent used and to state the temperature at which dissolution occurs. The amount of solvent is usually fixed at 100g. For example, the solubility of sugar (sucrose) at 20ºC is 240g in 100g of water. What is the maximum weight of sugar that will dissolve at 20ºC in a cup containing 350g of water? A saturated solution of a solute at a particular temperature is the one which will not dissolve any more of the solute at that temperature.
The solubility of a solute in water at a given temperature is the maximum amount of it that will dissolve in 100g of water at that temperature.
Dissolving a solid in water
Generally,
the solubility of a solute increases with increase in temperature.
However, there are a few exceptions e.g. the solubility of calcium
hydroxide decreases with increase in temperature. Sugar dissolves very
slowly in water at room temperature (20ºC). Stirring helps to make sugar
dissolve more quickly. But if you keep on adding sugar to the water
even with continuous stirring, eventually no more sugar will dissolve.
Extra sugar sinks to the bottom. The solution is saturated.
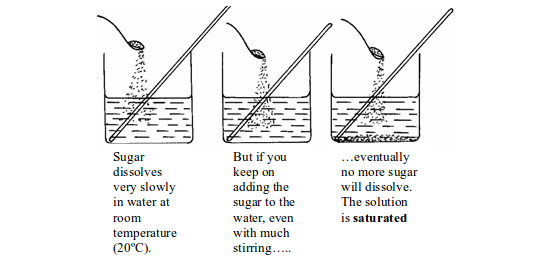
Dissolving a solid in water at room temperature
Now
let us look at what happens when you heat the sugar solution. If you
heat the solution up to 20ºC there is still undissolved sugar at the
bottom of the beaker. Increasing the temperature to 50ºC makes some
sugar dissolve but there is still some left. But if the temperature is
raised up to 80ºC all the sugar dissolves. You might even be able to
dissolve more sugar!
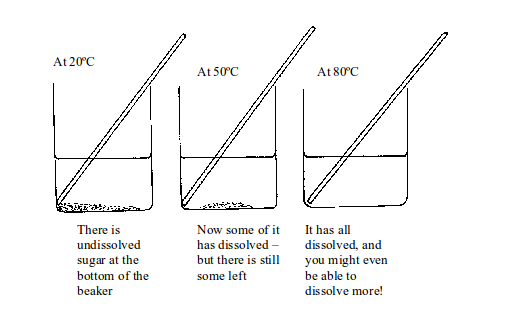
Dissolving a solid in water at higher temperatures
Therefore,
sugar is more soluble in hot than in cold water. In fact, this is
usually the case with soluble solids. If a solid is soluble in a liquid,
it usually gets more soluble as the temperature rises.
Solubility of different substances in different solvents
The solubility of a substance depends on the following factors:
1. The type of solvent used:
Iodine is slightly soluble in water. Only 0.3g will dissolve in 100g of
water at 20ºC. However, it is much more soluble in cyclohexane (organic
solvent). 2.8g of iodine dissolve in 100g of cyclohexane at 20ºC
2. The particles in it: Let us consider the dissolution of sodium chloride in water. When dissolved in water, the salt dissolves to form Na+ and Cl- ions. If sodium chloride is added to water, the Na+ ions will be attracted to the slightly negatively charged oxygen atoms of the water molecules whereas Cl- ions will be attracted to the slightly positively charged hydrogen atoms of the water.
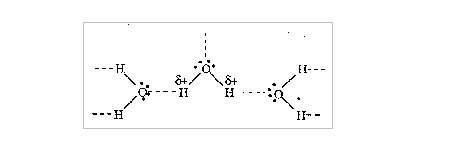
3. The temperature of the solvent:
As we saw early, the temperature affects the solubility of substances,
particularly solids. The higher the temperature the higher is the
solubility.
If
you shake some cyclohexane with a solution of iodine in water, almost
all iodine leaves the water and moves into cyclohexane layer. So,
cyclohexane is much better than water at separating iodine particles
from each other. The iodine particles are more attracted to cyclohexane
than they are to water. So, the solubility of each substance is
different. Look at these examples:
| Compound | Mass (g) dissolving in 100g of water at 25ºC |
| Silver nitrate | 241.3 |
| Calcium nitrate | 102.1 |
| Magnesium chloride | 53.0 |
| Potassium nitrate | 37.9 |
| Potassium sulphate | 12.0 |
| Calcium hydroxide | 0.113 |
| Calcium carbonate | 0.0013 |
| Silver chloride | 0.0002 |
As
you can see, one compound of a metal may be slightly soluble while
another is almost soluble (compare silver nitrate and silver chloride).
It depends on particles.
Measuring the solubility of a solid in water
Let us take potassium sulphate as our example. This is what to do:
- Put a weighed amount (say 2g) of potassium sulphate in a test tube. Add a little water from a measuring cylinder.
- Heat the test tube gently until the water is hot but not boiling. Add more water if necessary until the solid is just dissolved.
- Let the solution cool while stirring it with a thermometer. Note the temperature at which the first crystals form.

Measuring the solubility of a solid in water
Now look again at step 3.
If you add a little more water, heat the solution again to make sure
all the crystals have dissolved, and then let it cool, you will be able
to find the solubility at a lower temperature. You can repeat this for a
range of temperatures.
Calculating solubility
Since
you know the mass of solute and the volume of water you used, you can
work out the solubility as shown in the calculation below:
Example 1
2 grams of potassium sulphate were dissolved in 12.5 cm3 of water. On cooling, the first crystals appeared at 60ºC. What is the solubility of potassium sulphate in water at 60ºC?
Solution
12.5 cm3
of water weighs 12.5g. Also, remember that solubility is measured by
100g of water. If 2g of the salt dissolved in 12.5g of water, then the
amount of the salt in 100g of water.

Therefore, the solubility of potassium sulphate in water at 60ºC is 16 grams.
Solubility of gases
Solid
solutes usually get more soluble in water as the temperature rises. The
opposite is true for gases. Table 3.3 shows the solubility of different
gases in water at different temperatures.
Solubility of different gases in water
| Gas | Solubility (cm3 per 100cm3 of water) at..... | |||
| 0ºC | 20ºC | 40ºC | 60ºC | |
| Oxygen Carbon dioxide Sulphur dioxide Hydrogen chloride | 4.8171798050500 | 3.392.3425047400 | 2.556.6217044500 | 1.936.0-42000 |
Look at carbon dioxide. It is quite soluble in water at room temperature (20ºC). But when it is pumped into soft drinks under pressure, a lot more dissolves. Then when you open the bottle, it fizzes out of solution.
Look at hydrogen chloride. At room temperature, it is over 14000 times more soluble than oxygen.
Generally,
the solubility of gases changes with temperature and pressure. It
decreases with temperature and increases with pressure.
Solubility curves
The
solubility of a particular solid in water can be measured over a range
of temperatures up to 100ºC. The maximum mass of solid that will
dissolve in 100g of water is found at each temperature. The values at
each temperature can then be plotted to give a solubility curve. A curve
that shows how the solubility of a substance changes with temperature
is what we call a solubility curve.
Table bellow shows the solubility of some salts in water at different temperatures.
Solubility of some salts in water
| Temperature in ºC | Solubility in g of salt per 100g of water | ||
| Sodium chloride | Copper (II) sulphate | Potassium nitrate | |
| 10 | 38 | 18 | 20 |
| 20 | 38 | 20 | 30 |
| 30 | 38 | 24 | 44 |
| 40 | 38.5 | 28 | 60 |
| 50 | 38.5 | 34 | 80 |
| 60 | 39 | 42 | 104 |
| 70 | 39 | 50 | 152 |
For
most substances, solubility in water increases with increase in
temperature. Table above shows the solubility of some salts in water at
different temperatures.
When the values for each salt shown on the table are represented on a graph paper, different solubility curves result.
Look
at the values in table above again. On a graph paper, use the same set
of axes to plot solubility (vertical axis) against temperature
(horizontal axis). Draw a smooth best-fit curve for each salt.
- Which of the salts is the most soluble at 15ºC?
- Which of the three salts is the most soluble at 55ºC?
- At which temperature do sodium chloride and potassium nitrate have the same solubility?
The
curves in figure bellow show how the solubility of different salts
changes with temperature. You can see that the solubility of most solids
increases with increase in temperature. The increase for sodium
chloride is very small and almost negligible. The increase for the other
salts is as shown in the graph.
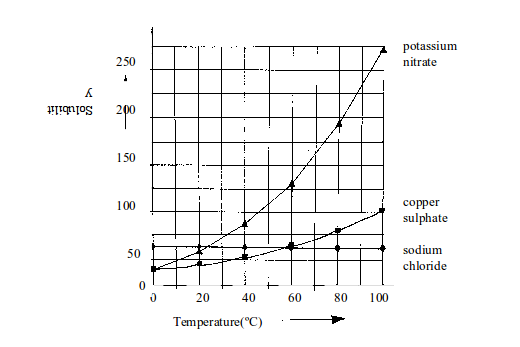
Solubility curves for three solids in water (solubility measured in grams of solid per 100g of water)
For
gases, the solubility decreases with increase in temperature. This
means that decreasing the temperature will increase the solubility of
gases. Figure 3.10 shows the solubility curves for some common gases.
Compare these curves with those for solids in figure above.
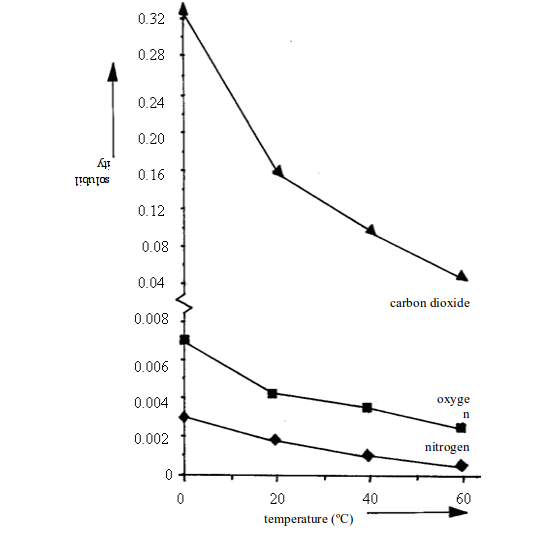
The solubility of three gases from the air in water (solubility measured in grams of gas per 100g of water)
Using solubility curves
Data can be obtained from the solubility curves in various ways. For example, look at figure above.
(a) What mass of potassium nitrate dissolves in 100g of water at
- 40ºC and
- 50ºC?
From the graph:
- At 50ºC, 137.5g of potassium nitrate dissolve in 100g of water.
- At 40ºC, 62.5g of potassium nitrate dissolve in 100g of water.
(b) What mass of potassium nitrate will crystallize out when a saturated solution in 100g of water is cooled from 50ºC to 40ºC?