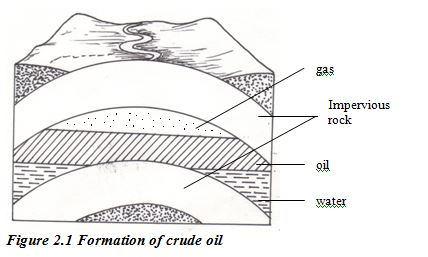
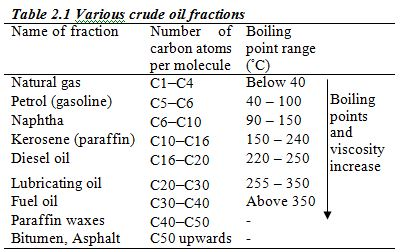
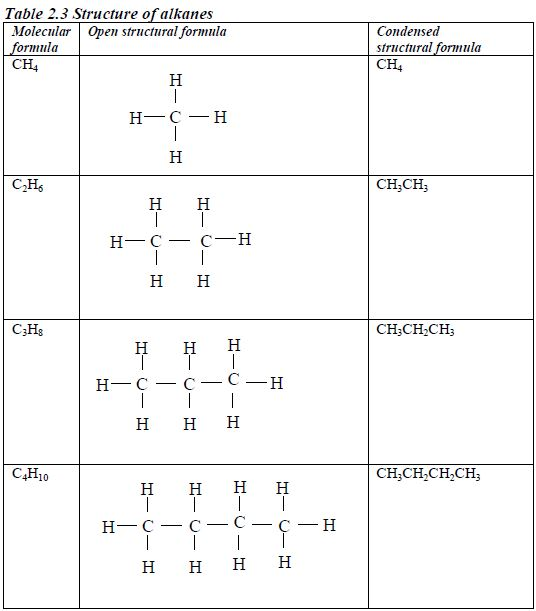
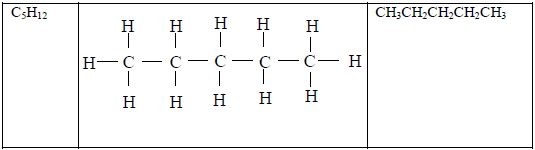
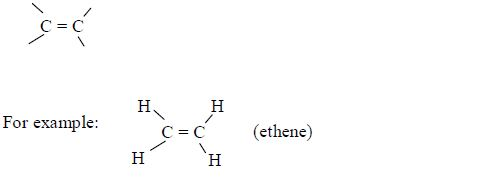
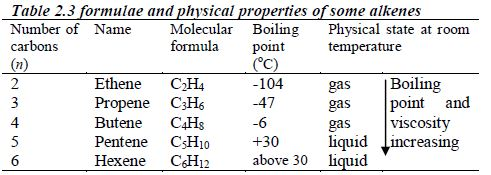
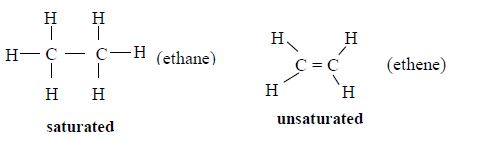
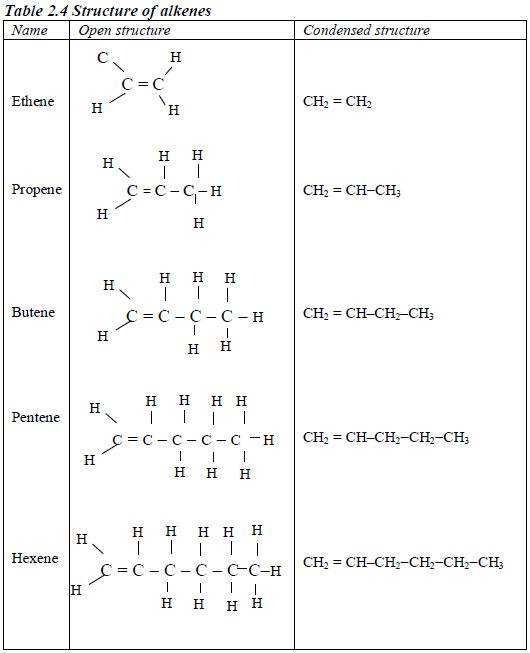
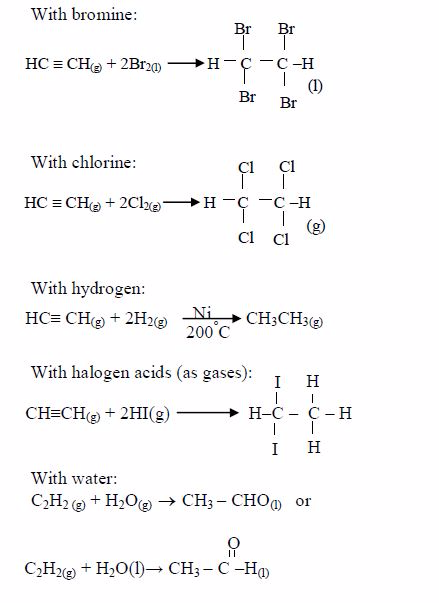
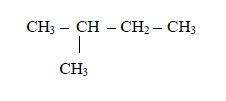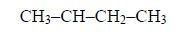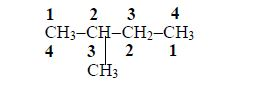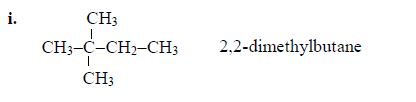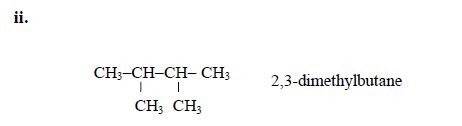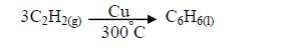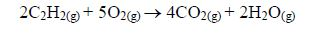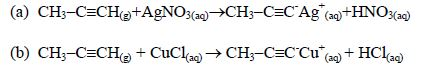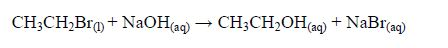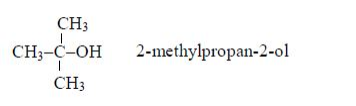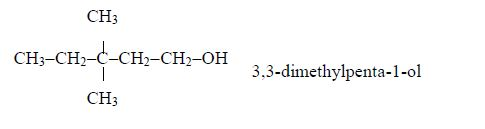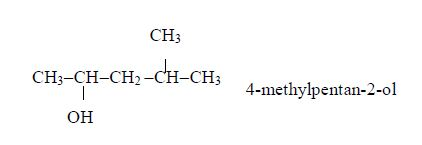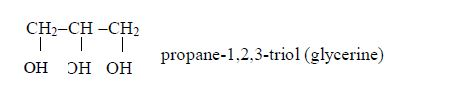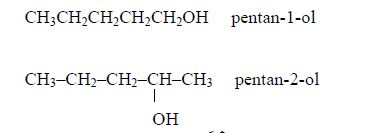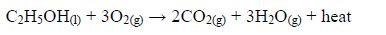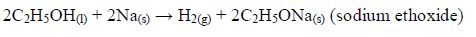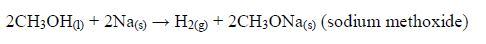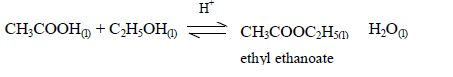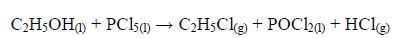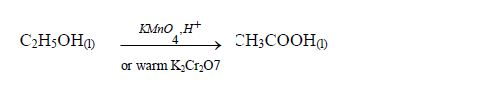ORGANIC CHEMISTRY
Introduction to Organic Chemistry
Carboxylic
acids form a homologous series of the general formula CnH2n+1COOH (or
CnH2n+1CO2H), where n = 1, 2, 4, etc. for successive members of the
group. All these acids have the characteristic functional (carboxyl)
group, –COOH, attached to a hydrocarbon chain.
Natural Sources of Organic Acids
There are various natural sources of organic acids. Some of these sources are:
- milk (lactic acid)
- citrus fruits (citric acid);
- tobacco (nicotinic acid); and
- tea (tartaric acid).
The Oxidation of Ethanol to Ethanoic Acid
Explain the oxidation of ethanol to ethanoic acid
When exposed to open air, ethanol is oxidized (by oxygen of the air) to ethanoic acid. The reaction for the process occurs thus:
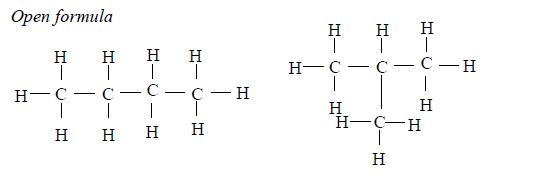
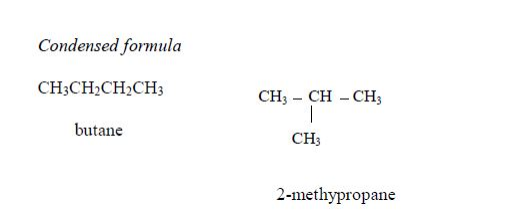
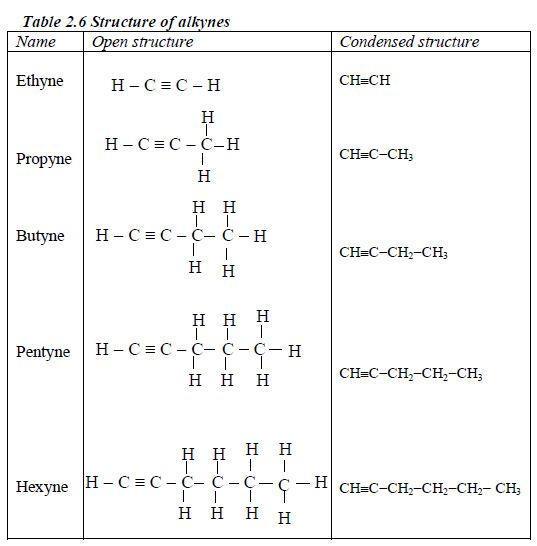
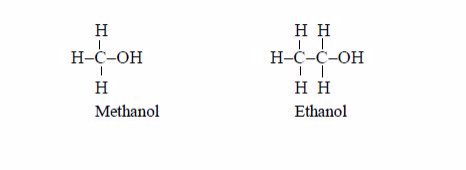
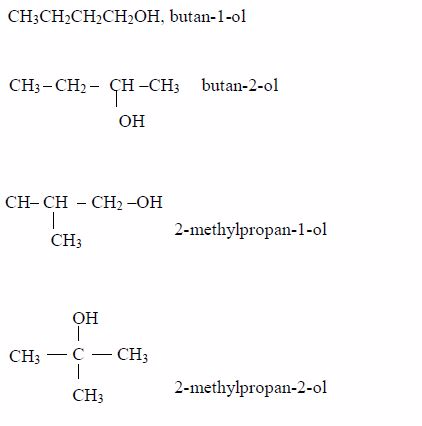
The Structures of the Homologues of Carboxylic Acids up to Five Carbon Atoms
Write the structures of the homologues of carboxylic acids up to five carbon atoms
Carboxylic
acids are named as if they are derived from alkanes by the replacement
of one hydrogen atom by the –COOH group. The two lowest members,
containing one atom and two carbon atoms respectively, are:

The other members of the homologous series are as shown below:
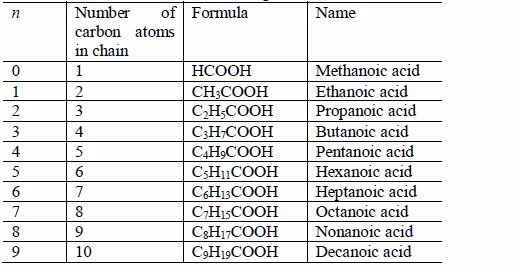
The
successive members of the series have molecular formulae which differ
by –CH2 It is important to remember that every carboxylic acid molecule
contains the functional group –COOH which is called the carboxyl group.
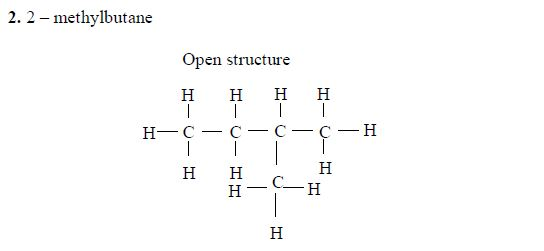
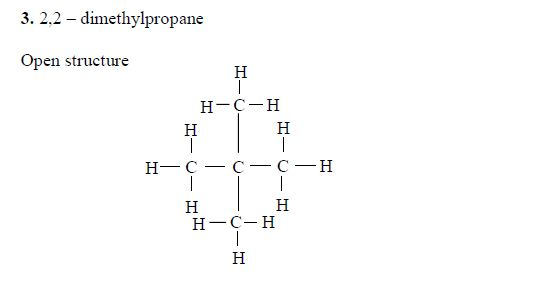
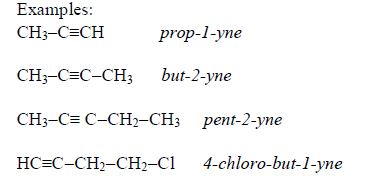
The Isomers of Carboxylic Acids up to Five Carbon Atoms
Like
other organic compounds, carboxylic acids also exhibit isomerism.
Isomers of carboxylic acid are a result of branching of the hydrocarbon
end (R) rather than the position of the carboxyl group in a molecule of
the carboxylic acid. More isomers of the carboxylic acids can be created
by branching the hydrocarbon end in as many different ways as possible.
Rules
The carbon of the carboxyl group (–COOH) is considered as carbon atom number 1.
Identify the positions of the alkyl group(s) attached to the (longest) acid chain. For example, in a molecule,
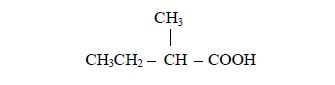
the alkyl group is methyl (–CH3) and it is attached to carbon number 2.
Name
the branched alkyl group, followed by the name of the acid to which the
alkyl group is attached. For example, in the case above (rule no.2):
- the alkyl group is methyl;
- it is attached to carbon number and
- the acid to which it is attached is butanoic acid,CH3CH2CH2COOH
Therefore, the name of the compound is 2-methylbutanoic acid.
In
case there occurs more than one alkyl groups in the compound the
prefixes di(2), tri(3), tetra(4) etc (as it was the case in alkanes) may
be used. For, example in the compound
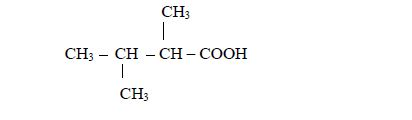
- there are two methyl groups, one attached to carbon number 2 and the other to carbon number 3; and
- they are both attached to butanoic acid chain.
Therefore, the name of the compound is 2,3-dimethylbutanoic acid.
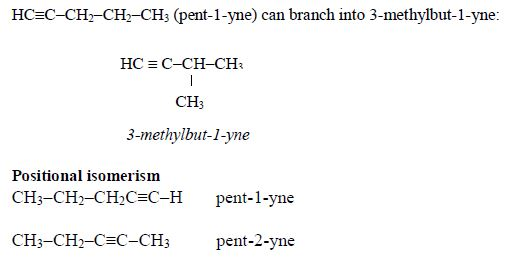
Isomerism and nomenclature
Branching
isomerism is found in this homologous series. Isomerism in carboxylic
acids begins from butanoic acid, C3H7COOH. The first three members of
the series do not show isomerism because their hydrocarbon ends do not
form branches. The following are the structures and names of the isomers
of carboxylic acids up to five carbon atoms:
- Butanoic acid, C3H7COOH or C3H7CO2H or CH3CH2CH2COOH
Isomers:
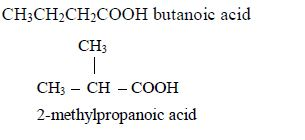
- Pentanoic acid, C4H9COOH
Isomers
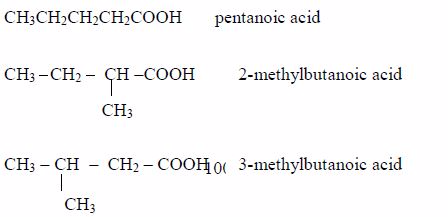
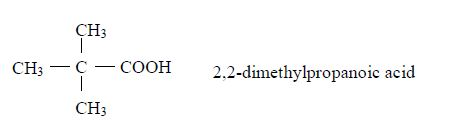
The Properties of Carboxylic Acids
Carboxylic acids are weak acids. They are slightly ionized in dilute solutions.
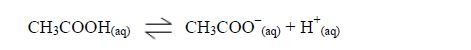
Like
inorganic acids, their solutions contain H+ ions. The presence of H+
ions give the solutions acidic behaviour, that is, their solutions
affect indicators, just like the inorganic acids do.
Neutralization
Like
inorganic acids, carboxylic acids react with metals, alkalis,
carbonates, and hydrogen carbonates to form salts. For example:

Esterification
The
reaction between carboxylic acids and alcohols is called
esterification. The acids will react reversibly with alcohols to form
sweet–smelling esters. Concentrated sulphuric acid is a catalyst for the
reaction.
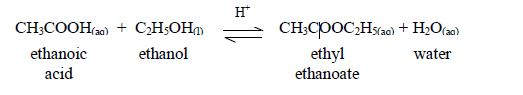
The
reaction can be reversed to recover an acid and alcohol again by
boiling the products (an ester + water) with a mineral acid (HCl or
H2SO4) or with an aqueous alkali (KOH or NaOH) as a catalyst.
Esters
are manufactured for use as solvents, food flavourings, and fragrance
for perfumes and beauty products. Ethyl ethanoate is just one example of
many esters. The esters usually have strong and pleasant smells. Many
of these compounds occur naturally. They are responsible for the
flavours in fruits and for the scents of flowers. Fats and oils are
naturally occurring esters used for energy storage in plants and
animals. Some of the naturally occurring esters include:
- vegetable oils e.g. palm oil, groundnut oil, cashewnut oil, olive oil, sunflower oil, etc; and
- animal fats.
All esters contain the functional group,

, where R is any alkyl group.
Preparation of Soap from Animal Fats or Vegetable Oil
Prepare soap from animal fats or vegetable oil
Vegetable
oils are formed from fatty acids and an alcohol called glycerol (also
called glycerine). Fatty acids are carboxylic acids with long chains of
carbon atoms. They are called “fatty” because the long chains repel
water, making them immiscible with water. Glycerol or glycerine (or
propane–1,2,3-triol) has three –OH groups. This is how fatty acids and
glycerol react:
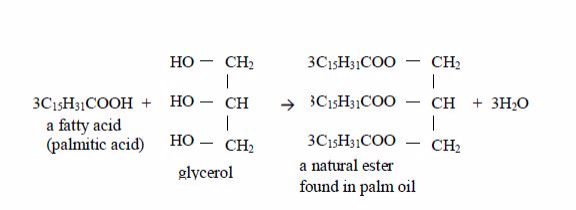
Preparation of soap from oils
Soap
is made by heating animal fats or vegetable oils with sodium hydroxide
solution. The oils react with the solution of sodium hydroxide and break
down to form glycerol and the sodium salts of their fatty acids. These
salts are used as soap. The reaction equation is:
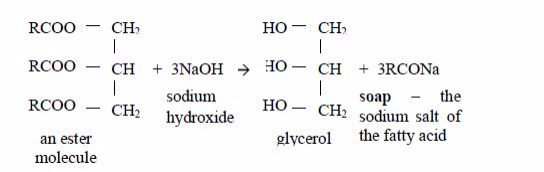
This
process is known as saponification. The soap you buy is made from a
blend of different oils. When soap dissolves in water it ionizes thus:
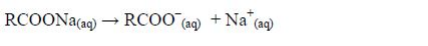
The cleansing agent in soap is the ion, RCOO–