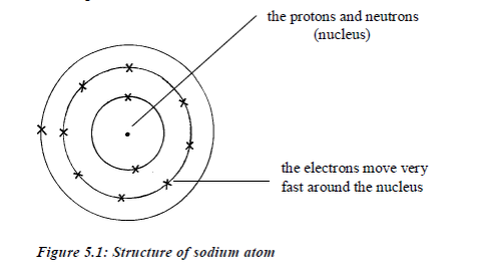
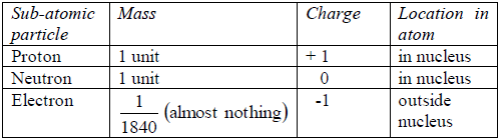
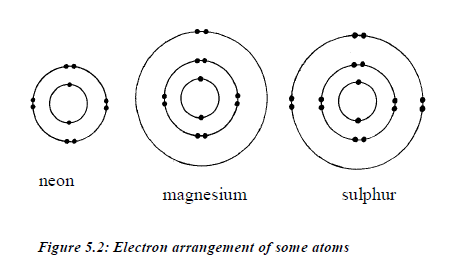
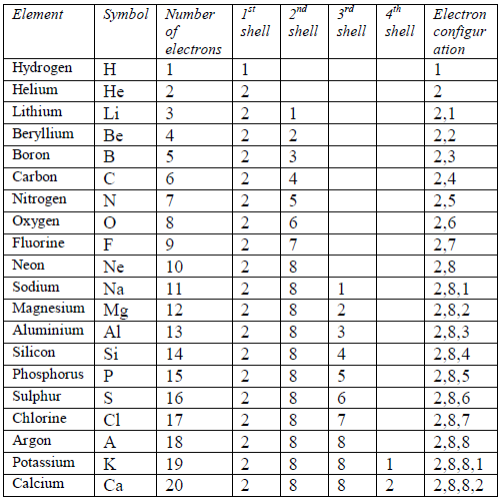
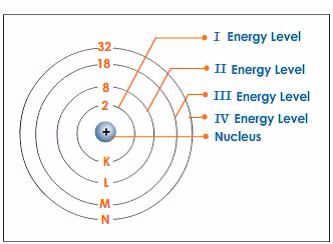
ATOMIC STRUCTURE
The Atom
Atomic number, Mass number and Isotope
Relationship between Atomic Number and Number of Protons
All
atoms of one element have the same number of protons. This is called the
atomic number (or proton number) of that element.It is given by the
symbol Z.
No
two elements can have the same atomic number. Sodium atoms have 11
protons. This is what makes them different from all other atoms. Only
sodium atoms have 11 protons, and any atom with 11 protons must be sodium
atom.
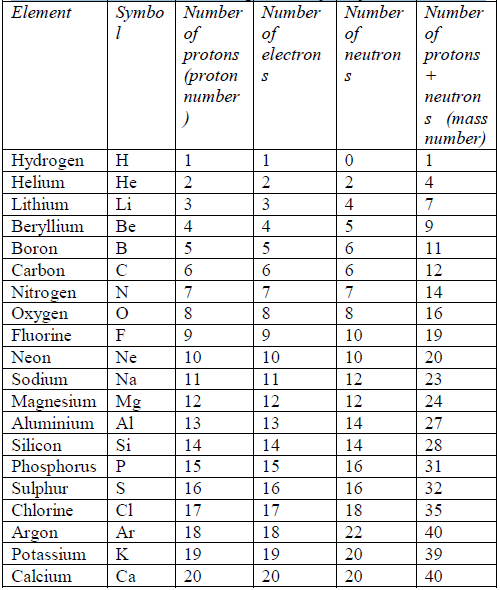
In the same way, an atom with 6 protons must be carbon atom.Also any atom with 7 protons must be nitrogen atom. So, you identify an atom by the number of protons in it. There are 109 elements altogether. Of these, hydrogen has the smallest atoms,with only 1 proton each. Helium atoms have 2 protons each.Lithium atoms have 3 protons each, and so on up to meitnerium atoms, which have 109 protons each. Table 5.3 shows the first 20 elements arranged according to the number of protons they have.
Every
atom has an equal number of protons and electrons, so the atomic number
also tells us the number of electrons in that atom.In any given atom of
an element, the number of neutrons has no effect on the identity and
properties of that particular element. It is the number of protons and
electrons that determine the identity and properties of any given
element. The number of neutrons only affects the mass, since each one of
them has the same mass as that of a proton.
Mass Number of an Atom from Numbers of Protons and Neutrons
Protons
alone do not make up all the mass of an atom. The neutrons in the
nucleus also contribute to the total mass. The mass of the electrons can
be regarded as so small that it can be ignored. As a proton and a
neutron have the same mass, the mass of a particular atom depends on the
total number of protons and neutrons present. This is called mass
number (or nucleon number). The mass number of an atom is found by
adding together the number of protons and neutrons. It is given by the
symbol A. Table 5.3 shows the mass number of the first 20 elements,
arranged in order of increasing atomic mass (mass number).If the mass number and atomic number for any given atom are known, then its sub-atomic composition can be worked out.
The mass number = number of protons + neutrons in an atom. Sodium atom has 11 protons and 12 neutrons, so the mass number of sodium is 23. Since the atomic number is the number of protons only, then:
Mass
number – atomic number = number of neutrons. So, for sodium atom, the
number of neutrons = (23-11) =12. You can also take into account the
fact that, because the number of protons is always equal to the number
of electrons, then the number of electrons in sodium atom is simply 11.
The same rule can be applied to work out the sub-atomic composition of
any element.
These two relationships are useful:
- Number of electrons = number of protons = atomic number
- Number of neutrons = mass number (A) – atomic number (Z).
The Concept of Isotope
Atoms
of the same element may have different numbers of neutrons. In a normal
situation, atoms of the same element will have the same number of
neutrons. However, many cases occur in which two atoms of the same
element contain the same number of protons but different numbers of
neutrons. Having equal number of protons, these atoms must also have
equal numbers of electrons. However, the differing numbers of neutrons
cause the atoms to have different mass numbers. An element showing such
properties is said to show isotopy and the varieties of the atom are
called isotopes of the element.
Therefore,
isotopy can be defined as the tendency of atoms of one element to
posses the same atomic number but different mass numbers (atomic
masses). Isotopes can be defined as atoms of the same element with the
same number of protons but different numbers of neutrons, or as „atoms
of the same element with the same atomic number but different atomic
masses‟.
The
isotopes of an element have the same chemical properties because they
contain the same number of electrons. It is the number of electrons in
an atom that decides the way in which it forms bonds and reacts with
other atoms. However, some physical properties of the isotopes are
different. The masses of the atoms differ, and therefore other
properties, such as density and rate of diffusion, also vary.
Many
isotopes (like tritium) are unstable. The extra neutrons in their
nuclei cause them to be unstable so that nuclei break spontaneously
(that is, without any extra energy being supplied), emitting certain
types of radiation. They are known as radioisotopes.
Notation for isotopes
In
order to distinguish between different isotopes of the same element in
writing symbols and formulae, a simple system is adopted. The isotope of
an element, say X will have the symbol X,AZ , where A is the mass
number of the isotope and Z is the atomic number of any atom of X. Thus,
for all isotopes of one element, Z is constant, and A varies because
there are different numbers of neutrons in the different isotopes of the
element. For example, the three isotopes of carbon are expressed as
12C6, 13C6,and 14C6. Chlorine has two isotopes: 35C17 and 37Cl7 .
Since A represents the total number of neutrons and protons in the
nucleus of an atom (mass number/atomic mass), and because Z is the
number of protons (atomic number), then the number of neutrons in the
nucleus of a given isotope is given by:Number of neutrons in the nucleus
= A – Z
Relative atomic masses
As
we have seen, most elements exist naturally as isotopes.Therefore, the
value we use for the atomic mass of an element isan average mass. This
takes into account the proportions(abundance) of all the naturally
occurring isotopes. If a particular isotope is present in high
proportion, it will make a large contribution to the average.
Example
A
sample of chlorine gas contains 75% of the isotope 35C17 and 25% of
the other isotope 37C17 . What is the relative atomic mass of chlorine?
Solution
To work out this problem, simply multiply the mass number of each isotope with the abundance and sum up the products thus:
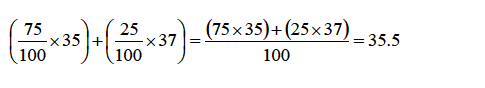
This
average value for the masses of atoms of an element is known as the
relative atomic mass (Ar).Therefore, the relative atomic mass of
chlorine is 35.5 (i.e., Ar =35.5).