THERMAL CURRENT ELECTRICITY
Simple cellis the cell consists of copper and zinc cathode with dilute sulphuric acid as Electrolyte.
The Mode of Action of a Dry Cell (Leclanche)
Describe the mode of action of a dry cell (Leclanche)
Action of simple cells;
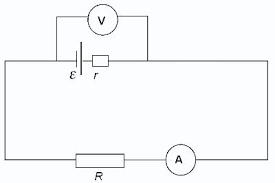
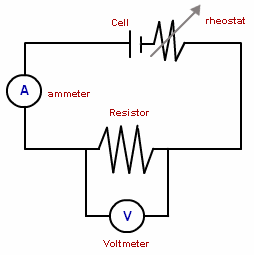
- At cathode: The zinc plate dissolves in the sulphuric acid solution and liberates electrons into the external circuit. The metal discus had to be of different material Volta used copper and zinc discs sand witched by cloth soaked in salt water. The combined device was called a voltaic pile. Volta also obtained the same effects by using copper and zinc plates dipped in dilute sulphunic acid. Volta called these devices, arranged in series. The “ Crown of cups” zn - 2e - Zn 2+ The 2n 2+ ions go into solution.
- At anode: Positively charged hydrogen lons (H+) are attracted towards the negatively charged copper plate2H + + 2e ----- H2.The chemical reaction in the cell creates a potential different between plates, causing electrons to flow when the two plates are joined with a wire. The electrons flow is maintained by the chemical change that occurs when the zinc dissolves in the sulphuric acid. Since simple cell, which is able to adrive an electric current through a circuit, is said to be a source of electromotive force (e.m.f)
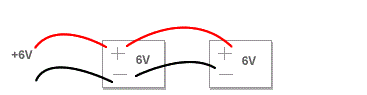
Voltage of Combination of Cells in Series and Parallel
Determine voltage of combination of cells in series and parallel
Connecting in Series
When connecting your batteries in Series you are doubling the voltage while maintaining the same capacity rating (amp hours). This might be used in a scooter, Power Wheels kids vehicle, or other applications. Just use a jumper wire between the negative of the first battery and the positive of the second battery. Run your negative wire off of the open connector from the first battery and your positive off of the open connector on your second battery.
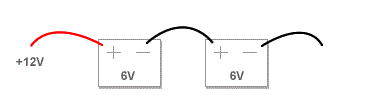
Connecting in series (double voltage, same capacity [ah]
Connecting in parallel
When connecting cells in parallel, you are doubling the capacity (amp hours) of the battery while maintaining the voltage of one of the individual batteries. This would be used in applications such as laptop batteries, some scooters, some ups backups, etc. Use a jumper wire between the positives of both batteries and another jumper wire between the negatives of both batteries. Connect your positive and negative wires to the same battery to run to your application.
Connecting in parallel (double voltage, same capacity [ah]
The Cell Defects
Identify the cell defects
Simple Have two main defects which cause the current to diminish quickly when the cell is being used.
Two defects of simple cell are:
- Polarisation
- Local Action
Polarisation
Polarisation is the defect occurs in simple cell caused by the formation of hydrogen bubbles around the copper plate.These bubbles insulate the copper plate and prevent other positive hydrogen ions from receiving electrons from the copper plate to become neutral.
Also hydrogen ions accumulating at the copper plate repels other Hydrogen ions ( This defect is called Back e.m.f and opposes or weakens the main e.m.f of the cell).
How to minimize polarisation
- Polarisation can be minimised by using suitable oxidising agents, called depolarisers, to remove the hydrogen. An example of depolarisers for hydrogen is potassium dichromate. The dichromate oxidizes the hydrogen to form water.
Local Action
Local action is the process by which a cell is used up when no external current is flowing.Commercial zinc normally contains atoms of Iron, lead, carbon, etc called impurities.
When commercially zinc is used in a simple cell, bubbles of hydrogen are seen escaping from the zinc plate (evidence of local Action).The Impurities on the surface of the Zinc act as a second plate of a cell. As a result, Zinc dissolves in the acid even when the cell is not in use (This process wastes the zinc)
How to minimize local action
The problem of local action can be overcome by using amalgamated zinc plate (zinc coated with mercury).
Pure zinc in mercury form zinc amalgam.This is done by rubbing some mercury on the zinc plate.
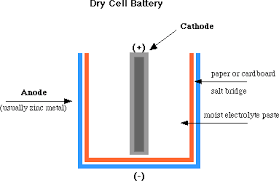
The leclanche cell:Is the cell uses an aqueous solution of ammonium chloride (sal ammoniac) as the electrolyte, amalgamated zinc rod as cathode. The carbon rod as anode fixed in a porous pot, containing a powdered mixture of carbon and manganese (iv) oxide.
The carbon makes the mixture more conducting, and the manganese (IV) oxide (manganese dioxide,MnO2) acts as a depolarizer.
The Dry cell
Is a modified leclanche cell in which the main electrolyte is a paste of starch and ammonium chloride. The action of the cell is similar to that of the wet leclanche cell.
The paste is prevented from drying by sealing the top of cell with some insulating materials. This type of cell gives a larger current and have a shorter recovery (demoralising time) than the 'wet’ type;
Hence it is useful for a greater variety of applications.However, Local action cannot be eliminated completely in these cells, so that the cells have a storage (or shelf) life ranging from a few months to up to several years if stored in a cool place
The leclanche cell is called a primary cell and this type of cell current is produced from a non recoverable or irreversible chemical reaction.
Example:When all the zinc has been dissolved in the simple cell it can never be recovered to its original form by passing a current thought the cell in the opposite direction.
Secondary cell
This is the cell which can be recharged after it hasrun down (used). This is done by passing a d.c current from a dynamo or similar device through the cell in the opposite direction to that in which the cell usually supplies current in an external circuit.
Also called storage cells or accumulator some common accumulators are:
- Lead Acid accumulators
- Nickel – Cadmium accumulators
- Alkaline and chloride accumulators
The main advantage of this type of cell is that it has a very low internal resistance and can therefore give a large current with very little drop in the terminal potential difference.
The Mode of Action of Lead-acid accumulator
Describe the mode of action of lead-acid accumulator
The lead – acid accumulator cell consists of two plates of lead immersed in sulphuric acid.The acid is in a plastic container.Two or more cells may be connected to forms battery
The positive terminal is lead (iv) oxide and the negative terminal is lead.
Wood / rubber/ separator/Insulator
Cathode electrode: Lead plate (–)
Anode Electrode:lead (iv) oxide (+)
Before the accumulator is use it has to be charged
The Charging and Discharging Phenomenon of an Accumulator
Explain the charging and discharging phenomenon of an accumulator
Charging is done by connecting across its plates as source of direct current. If the current of 2A may be allowed to flow across the terminal of the cell. The positive terminals of the cell and the source of current (dynamo) must be connected together similar the negative terminal must be connected together.
If the cell is now fully charged the p.d across its terminals on open circuit is 2v, and the cell is ready for use.It can light says a2v electric lamp connected across its terminals. The currents will flow in the reverse direction to the charging current.
Discharging Accumulator
- Is the process of using charges stored in the lead – Acid Accumulator.
- When the accumulator is discharged after long use both plate become coated with lead sulphate.
- The relative density (R.D) of Acid also become less and the P.d of cell falls.
- Recharging will restore the plate of lead (iv) oxide and lead, and the relative density of acid will rise to 1.25
- The accumulator must not allowed to discharge below the stated values of p.d and Relative density, or it will not be possible to recover it on recharging.
- The capacity of an accumulator is the amount of current in amperes that the cell can send thought a circuit is measured in ampere – Hour (Ah)
- The charging rate an Accumulator is a current in amperes, numerically equal to one – tenth (1/10) of the capacity required in Recharging.
- Lead-acid accumulators have high e.m.f (2v per cell) and allow internal Resistance.
- Accumulators are best cared for by a regular check of the level of the sulphuric acid.
- Any loss due to evaporation must be replenished with distilled water only.
- No acid should be added unless there has been same spillage from the cell.
- Accumulators must be recharged regularly using the charging current recommended by the manufactures.
- They should not left in discharged condition for a long time.
- When not in use they should be recharged at least once every month.
- An accumulator should never be short-circuited.
- Shorting the cell may cause swelling and buckling of the plates due to excessive heat developed in the cell, leading to permanent damage (A cell in this condition is said to be sulphated).
Cells and Accumulators in Daily Life
Use cells and accumulators in daily life
Uses of electric cells
- Electric cells are very useful when no mains supply of electricity is available or when connecting to a main supply of electricity would be inconvenient.
- Portable radios, torches, calculators and watches are example of devices that use primary cells.
- It is possible to buy rechargeable batteries for these devices.These are secondary cells to start the engine and to run all the electrical circuits.
- This cell is recharged by the alternator when the car is in use
- Thank you