VAPOUR AND HUMIDITY
- These are molecules which escape into the atmosphere after liquids are heated.
- When a liquid is heated strongly then molecules tends to escape ( those molecules are called vapour).
- Most liquids evaporates at any temperature however liquids may vary in the rate at which they evaporate at ordinary temperature.
- Alcohol and ether evaporate rapidly but lubricating oil and mercury hardly evaporate.
- Evaporation of a liquids result in the formation of vapour.
Factors Affecting Evaporation of a Liquid
Identify factors affecting evaporation of a liquid
There are several factors which affect evaporation of liquids when heated which include the following:
- Nature of the liquid: Normally liquids evaporation differs depending on the nature of liquid. Example; Volatile liquids evaporate faster than non-volatile liquids, which evaporate slowly. Alcohol evaporates faster than other liquids like water. The boiling point of alcohol is 780C while that of water is 100oC.
- Pressure above the liquid (atmospheric pressure): When the atmospheric pressure is high, the rate of evaporation may be reduced.
- Surface energy of the liquid: This forms a boundary or skin between the liquid and the atmosphere. The surface energy prevents molecules with lower kinetic energy from escaping into the atmosphere. Some liquids such as alcohol have low surface energy , hence they evaporate rapidly.
Question Time 1
Why do molecules escapes when the liquid is heated?
When the liquid is heated, the molecules tend to gain (absorb) kinetic energy hence the random speed of the moleculesincreases.
The process of evaporation of liquid can be explained using Kinetic Theory.When a liquid is left to evaporate in a closed container, the pressure of the vapour in the container gradually increases.
Difference between Saturated and Evaporation of a Liquid
Distinguish between saturated and evaporation of a liquid
Vapours
The molecules in a liquid are in a state of continuous motion and some of those at the liquid surface will gain sufficient energy to escape from the surface altogether. The molecules that have left the surface are said to be in the vapour state. The difference between a vapour and a gas is purely one of temperature, a vapour being a gas below its critical temperature.
This phenomenon is known as evaporation. The number of molecules leaving the surface, and hence the rate of evaporation, will increase with temperature as the liquid contains more energy at a higher temperature. The effect of the evaporation of a liquid can be shown clearly by the following experiment.
Some ether is run into the flask, as shown in the figure below. It will evaporate in the enclosed space and the pressure that it exerts on the water will force a jet of water out of the tube. Warming the liquid will increase this evaporation and give a more powerful jet.
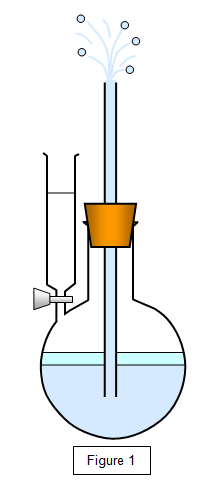
You can show that the rate of evaporation may be increased by:
- Warming the flask gently.
- Increasing the area of the liquid surface.
- Blowing a stream of air across the surface.
- Reducing the pressure above the liquid surface.
Saturated vapours
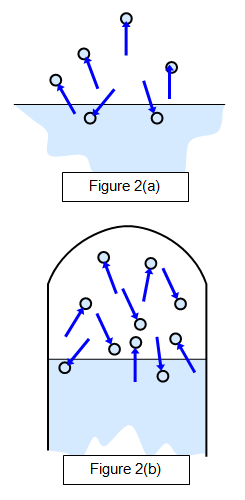
When a liquid is in a closed container the space above the liquid is full of vapour, and the vapour is then described as a saturated vapour - this means that the density of the liquid molecules in the air is a maximum. This is due to molecules continually escaping and reentering the liquid. At any moment the number of molecules leaving the surface will be equal to the number returning to it and so a dynamic equilibrium is set up.
The properties of saturated vapours were first investigated by Dalton around 1800. This is shown in Figure 2(a), which shows a state before saturation has been reached (when there will be more molecules leaving the surface than returning to it) and Figure 2(b), which shows the saturated state. A dynamic equilibrium exits here.
This vapour will exert a pressure and if there is sufficient liquid the air above the liquid surface will be saturated with vapour; the pressure that this saturated vapour exerts is known as the saturated vapour pressure (s.v.p.) of the liquid at that temperature.
Notice that since the velocity of the molecules increases with temperature the saturated vapour pressure also increases with temperature, and therefore the temperature of the vapour must be specified when quoting its saturated vapour pressure (s.v.p.)
The Effect of Temperature on Saturated Vapour Pressure (S.V.P) of a Liquid
Explain the effect of temperature on saturated vapour pressure (S.V.P) of a liquid
Saturated vapour pressure (S.V.P):Is the pressure exerted by vapour when a liquid is heated and reaches a state of Equilibrium where eventually the rate at which the molecules leave the liquid is equal to the rate at which others return to it.
The Height, of mercury represents the saturated vapour pressure of the liquid in the flask. Saturated vapour pressure increases with the increase in Temperature (Ti) and the increased with decrease in Temperature (Td).
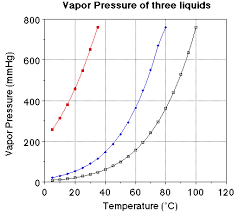
The graph of saturated vapour pressure (svp) against Temperature
The graph shows as saturated vapour pressure (s.v.p) increases then the temperature will increase and vice versa for decrease of temperature.
Boiling point (B.P):Is the temperature reached where the saturated vapour pressure (S.V.P) is equal to external atmospheric pressure.
The Boiling point of alcohol is 78OC water is 100OC and pressure of the atmosphere as 76cm of mercury.The intersection of the normal atmosphere pressure line with the liquids S.V.P curve.
Humidity
The Concept of Humidity
Explain the concept of humidity
HUMIDITY is the measure of the extent to which the atmosphere contains water vapour (moisture).
The Formation of Dew
Explain the formation of dew
DEW:These are deposits formed when the temperature fall slowly in the drops of water vapour.
Dew point (D.P): Is the temperature to which air must be cooled to become saturated. For example in an air container, if water vapour at pressure of 8mm of mercury were to be cooled , dew would form at 7.90C.
Measurement of Relative Humidity
Measure relative humidity
Absolute humidity is the mass of water vapour present in a unit volume of it and is usually expressed in grams per cubic metre (g/m3). The absolute humidity is not very frequently used since in practice we're more often concerned with the degree of wetness of the air.
Relative Humidity (R.H) is the ratio of the mass of water vapour actually in a unit volume of air to that is required to saturate it at the same temperature.
R.H = Mass of water vapour/Volume of air required to saturate the air at the same temperature
It is common practice to quote relative Humidity as a fraction or a percentage.
Thus
R.H = m/M X 100%
Where
M = mass of water vapour actually present in a unit of given volume of air.
M= mass of water vapour required to saturate the air at the same temperature.
Relative Humidity is also defined as the saturation vapour pressure of water at the dew point divided by the saturation vapour pressure of water at the original air temperature.
R.H = s.v.p at dew point/s.v.p at air Temperature
Note:The low value of relative humidity of air means that evaporation takes place readily from the surface of water.The high value of relative Humidity then evaporation does not take place readily from the surface of water.
Humid atmosphereis saturated with water vapour.
Perspiration: Is the evaporation of sweat from the skin, is not so effective at cooling the body in humid atmosphere.
Note:
- Cotton manufacturing industries are constructed on sites where the relative Humidity (R.H) Is High.
- Cotton fibres must not become too dry, otherwise they become Brittle and hence cause difficulties in spinning.
- In contrast, a dry atmosphere is required by ware House for the storage of food, Tobacco and assembling of certain electrical components.
- Air – conditioning plants are installed in ships and buildings for the purpose of moderating Humidity.
MEASURING RELATIVE HUMIDITY
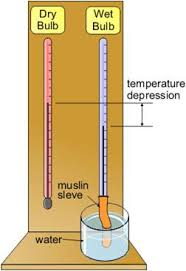
Hygrometers are instruments used for finding relative humidity at a given place. With most hygrometers, the relative humidity is determined by first finding the dew point and then using vapour pressure tables.
A very common type of Hygrometer (often known as Mason’s hydrometer). A piece of muslin wick is wrapped round the bulb of one the thermometer and its lower and dipped into water capillary action keeps the Muslin wrapped around the bulb wet.
- Evaporation of water surrounding the wet bulb absorbs heat from the bulb, consequently the temperature of the wet bulb falls.
- The reading of the wet bulb thermometer is normally found to be several degrees below that of the dry bulb Thermometer is known as the wet bulb depression.
Example 1
If the dry the temperature is 300C when the wet bulb temperature is 20OC then the wet bulb depression is 10oC the rate Evaporation depends on the amount of water Vapour present in the air.
The less moisture the air has the greater the difference between the two thermometer reading.The difference is therefore greatest for dry air and zero for saturated air.
REGNAULT’S HYGROMETER:
Is used to determine dew point and relative Humidity.Simplified form of regnault’s hygrometer consist of two test – tubes A and B with silvered ends C and D Respectively.
- Test tube A contains ether and it is fitted with rubber stopper which carries a thermometer T and two narrow tubes E and F.
- Test tube B. is empty and serves as comparison.
- Air is bubbled through ether via the narrow tube E by applying a fitted pump at the end of the Narrow tube F. This causes the rapid evaporation which results in the absorption of latent heat from ether and the container.
- Air surrounding the tube cools to temperature at which the water vapour present is sufficient to saturate it.
- Consequently is seen to form on C while D appears unchanged.
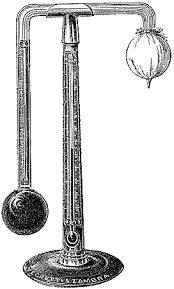
Above show Regnault (dew point ) hygrometer.
- The reading of the thermometer T is then noted.
- The flow of air through the ether is then stopped and the apparatus allowed warming up.
- The temperature at which the dew on C disappears is noted.
- The dew point is then taken as the mean of the two temperatures.
- Suppose the dew pint is Q1 and the actual air temperature is Q2 if the tables the value of S.V.P for water at Q1 and Q2 are X and Y millimeters of mercury Respectively.
- R.H = S.V. Pat Q1 x 100%/S.V.P at Q2
Example 2
The dew point in a room at a temperature of 100c is 12,55C if the saturated vapor pressure if saturated vapor pressures at these two temperatures are 15.5mm and 10.9mm of mercury respectively calculate the Relative Humidity.
Solution
Required: To find relative humidity, RH.
RH = S.V.P at dew pointx 100%/S.V.P at air temperature
RH= 10.9/15.5× 100%
Relative Humidity, RH = 71%
The Knowledge of Humidity in Daily Life
Apply the knowledge of humidity in daily life
Water in the atmosphere exists in different forms example: Clouds, Rain, Snow, Hail stones, Mist, Fog and Smog.
Clouds.
- Consist of the tiny droplets of water or Ice floating in the sky formed by condensation of water vapor in the upper atmosphere.
- Clouds formation occurs when temperature falls below the dew point and small particles of dust or salt crystals are present to act as nuclei on which condensation can begin.
- Cooling is due to upward movement of air accompanied by example.
- Clouds may also formed when a warm moist air current meets a cold one if the drops become big enough by joining together they may fall as rain.
Rain: These are drops of water that fall on the grounds when cooling occurs in the clouds.
Snow: Formed when the dew points is below the freezing point (F.P) 0OC.Under these conditions, the atmospheric vapour condenses directly into ice crystal.
Hail stones
- Formed due to super cooling of water droplets in such a way that the droplets are cooling below 0oC without freezing.
- When these droplets are carried upwards by ascending air currents, they solidify upon coming into contact with ice crystals in the upper atmosphere.
- Hail stones are dangerous to air craft and human beings.
Mist: Is the condensation of vapour Iito water droplets occurring near the ground.
Fog: Is a mist In which Visibility does not extend beyond 1km.
Smog:This is dense fog, where visibility is reducing to a few metres.
Thank you.
Thank you.




