HEAT SOURCES AND FLAMES
Types of flame
Flames
are formed by burning gases or vapours. During burning, heat and light
are given out. For any solid or liquid to burn with a flame, it must
first turn into inflammable vapours (gaseous state).
Luminous and Non-luminous Flames from Different Types of Flames
A
flame can be luminous or non-luminous. Flames of a candle and any oil
are usually smoky and luminous. Flames of such kind are normally of
little laboratory use. This is because they are not hot enough and would
deposit soot on laboratory apparatus. Coal gas also burns with a smoky
and luminous flame. With a Bunsen burner, one can produce two types of
flames namely, the luminous and non-luminous flames.
Luminous flame
This
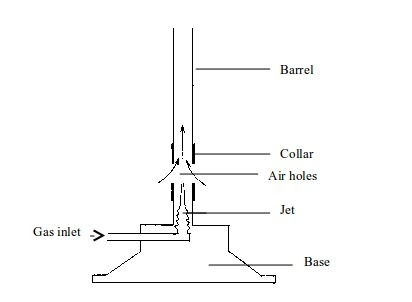
is a type of flame produced when the air holes of a Bunsen burner are
closed. When the air holes are closed very little air enters the barrel
of the burner. In this case, the flame will be large, unsteady and
bright
The flame will have four main zones each having a distinct colour.
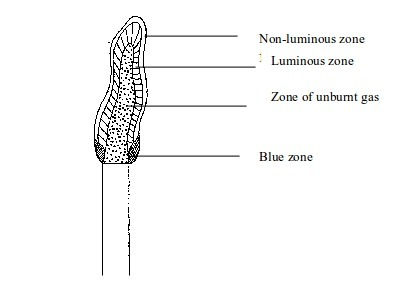
Luminous flame
- The inner dark zone - This is dark, cool and contains unburnt gas
- Luminous yellow zone - The gas burns in this zone but because the air is not enough the burning is incomplete. This leads to formation of tiny carbon particles from the gas. When these particles are white-hot, they result in formation of light (the yellow colour we see). If a cold evaporating dish, porcelain crucible, or glass is placed in this zone, it will blacken due to deposition of carbon particles (soot) on it.
- Outer zone - This is a non-luminous zone where the burning of the gas is complete due to presence of enough air. Because of the absence of carbon particles, this zone does not give out light. Consequently, the zone cannot be seen easily.
- Blue zone – Due to rising convectional current, there is sufficient supply of air for complete burning at this zone.
Non-luminous flame
When
air holes are fully opened, sufficient air enters the Bunsen burner
barrel and mixes well with the coal gas. Hence, the burning of the gas
is much quicker and complete. The flame is smaller and hotter.
Due
to absence of white-hot carbon, no light appears. The flame is
therefore non-luminous. The flame has three district zones each with a
different colour.
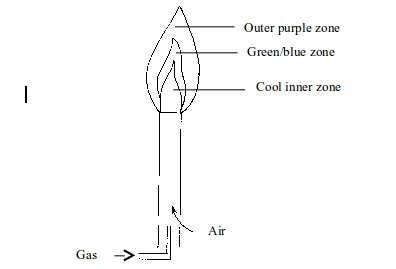
Non–luminous flame
- Cool inner zone – this is a zone of unburnt gas.
- Green/blue zone - part of the gas burns in this zone because there is not enough air to burn all the gas completely. However, no carbon is formed. The hottest part of the flame is at the tip of this zone.
- Outer purple zone – Burning of the gas in this zone is complete.
Major differences between luminous and non-luminous flames
| Non luminous flame | Luminous flame | |
| 1. | Formed when air holes are open | Formed when air holes are closed |
| 2. | Very noisy | Silent or calm |
| 3. | Comprises of three zones | Comprises of four zones |
| 4. | Forms no smoke or soot on apparatus | Forms a lot of smoke or soot on apparatus |
| 5. | Blue and almost invisible | Bright yellow and clearly visible |
| 6. | Very hot flame | Not a hot flame |
| 7. | Not bright | Very bright |
| 8. | Triangular flame | Wave-like flame |
Investigation of different parts of a flame
We can easily find out whether or not the inside of a flame is cool. Two experiments can prove this:
- (a) When a piece of cardboard is held horizontally over a non-luminous flame, we notice a burn mark as shown below:
When
held vertically over the flame, the burn mark is as shown in above.
Note that when performing this experiment, the cardboard should be
withdrawn from the flame just before it catches fire. We find that the
middle part of the cardboard does not get burned. This is the part in
the zone containing unburnt gas.
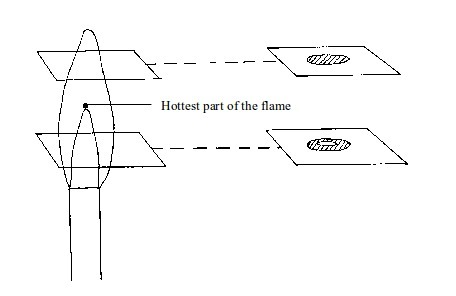
Burn mark on cardboard when held horizontally
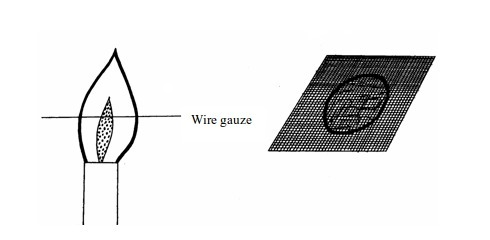
(b)
If the above experiment is repeated using a wire gauze, we notice that
the part in the middle will not become red hot except when the gauze is
held in the flame for a long time.
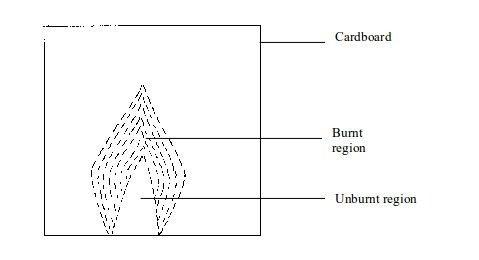
Burn mark on cardboard when held vertically
We
can prove the presence of unburnt gas in the Bunsen flame. This can be
done by inserting a glass tube into the flame as shown in figure bellow
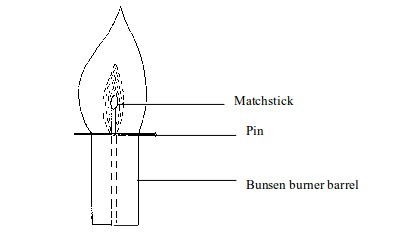
The
unburnt gas can be shown to have risen up the tube by putting a light
at the top of the tube. The flame will form. This indicates the escape
of unburnt gas through the tube.
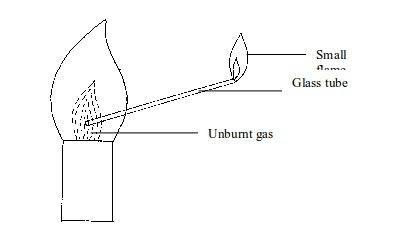
To indicate the presence of unburnt gas in a Bunsen burner flame
Uses of flames
Flames are used for different purposes. Some uses of the flames include the following:
- Production of heat for heating substances in the laboratory: In this case, a non-luminous flame, which produces much heat, is used. However, for reactions that require little heat, a luminous flame, which is not very hot, can be used.
- Flame tests for elements: In chemical analysis of some elements, a flame test is one of the preliminary tests normally used to identify an element. When some elements are strongly heated, they produce characteristic flame colours that distinguish them from one another. A non-luminous flame is often used.
- Production of light: Flames produce light that can be used to light a dark room. Therefore, an experiment that involves heating can even be conducted in the dark. The same flame is used to give heat as well as light. Here, a luminous flame is used. Examples of heat sources, which produce flames that may be used for lighting, are hurricane lamp, tin lamp, spirit lamp and candle.
- Cooking: Since it gives a hot flame and produces no soot, a non-luminous flame can be used for cooking food. Gas cookers, gas stoves and kerosene stoves usually produce such flames.
- Welding: A non-luminous flame is suitable for welding because it is very hot. In most welding operations, an oxyacetylene gas, a mixture of oxygen and ethyne, is used. When burned, the gas produces a flame hot enough to cut or melt the metal.