Concept of matter
Explain the concept of matter
Matter
is anything that has mass and occupies space. Therefore, anything that has real physical existence and is tangible such that can be seen and touched is termed as matter. Matter includes all materials around us such as water, soil, plants, animals, air, clothes and any other tangible materials you know.
What is substance?
What is substance?
Substance refers to any particular kind of matter. Substances include elements and compounds.
An element is a single pure substance that can be split into two or more constituents by any chemical means. When two or more elements are combined chemically, a compound is formed. The matter is made up of atoms, ions or molecules. You will learn more about this later.
An element is a single pure substance that can be split into two or more constituents by any chemical means. When two or more elements are combined chemically, a compound is formed. The matter is made up of atoms, ions or molecules. You will learn more about this later.
States of matter
There are Three States of Matter namely:
- solid-state
- liquid state and
- gaseous state
So,
each of the many millions of substances around us can be classified as a
solid, a liquid or gas. Look around you and name substances that are solids, liquids and gases. The state in which any matter exists depends on temperature and sometimes pressure conditions. One substance may exist as a solid in one condition and as a liquid or gas under a
different condition. Water is an example of such substances. This change is called a change in the state of matter.
The three physical states of matter differ in the way they respond to temperature and pressure. All three states can increase in volume (expansion) when the temperature is increased. They decrease in volume (contraction) when the temperature is decreased. Gases are easily compressed. Liquids are only slightly compressible. Solids are incompressible. They are not affected by the change in pressure.
How one may investigate the compressibility of solids, liquids and gases
Procedure
- Take three new syringes and fill them with sand, water and air respectively as shown in the figure below.
- Try to push at the end of each syringe.
- Observe what happens.
Figure
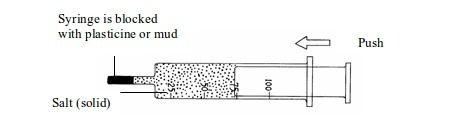
Compressibility of solids, liquids and gases
Observation
Which of the substances under investigation can compress into a smaller volume?
Findings
You should have found that a solid (sand) and a liquid (water) cannot be compressed but a gas (air) is easily compressed.
The three states of matter differ in their physical properties. These differences in properties are summarized in the table bellow
Differences in properties of the three states of matter
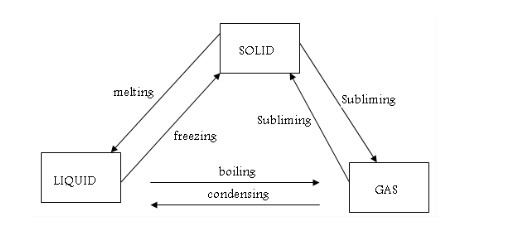
Change one state of matter to another
We
have seen that matter exists in three different states - solids,
liquids and gases. We can use the kinetic theory of matter to explain how a substance changes from one state to another. Basically, changes from one state to another are caused by alterations in temperature and pressure. Normally molecules, ions or atoms of a substance move faster when the temperature is increased.
Melting and freezing
Melting
is a change from solid to liquid state. When solids are heated, their constituent particles (atoms, molecules or ions) get energy and vibrate more violently. Vibrations of these particles overcome (exceed) their binding forces. The particles become mobile. The crystalline structure of solid is destroyed. A liquid state is reached and the particles are free to move. The temperature at which this happens is called the melting point of the solid.
The
the melting point of a solid tells us something about the strength of forces holding its constituent particles together. Substances with high melting points have strong forces between their particles. Those with low melting points have weak forces between their particles.
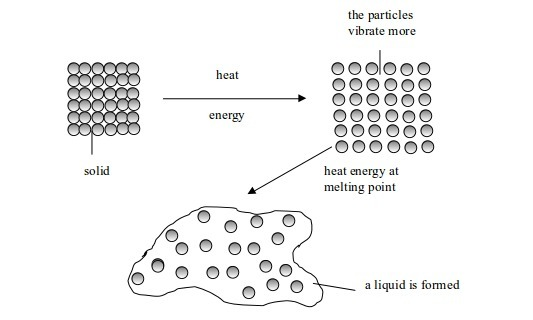
Change in state from solid to liquid
Freezing
is a change from liquid to solid-state. Freezing is the opposite of melting. The process is
reversed at the same temperature if a liquid is cooled. The temperature at which a substance turns to a solid is called freezing point. The melting point and freezing point of any given substance are both the same. For example, the melting and freezing of pure water take place at 0°C. Melting is not affected by any changes in atmospheric pressure.
reversed at the same temperature if a liquid is cooled. The temperature at which a substance turns to a solid is called freezing point. The melting point and freezing point of any given substance are both the same. For example, the melting and freezing of pure water take place at 0°C. Melting is not affected by any changes in atmospheric pressure.
Evaporation and boiling
Boiling
is a change from liquid to vapour state at a particular temperature.
Evaporation is the change from liquid to vapour state at any given temperature. If a liquid is exposed to open air, it evaporates.
Splashes of water evaporate at room temperature. After the rain, small pools of water dry up. When a liquid changes into a gas at any temperature,
the process is called evaporation.
Evaporation takes places from the surface of the liquid. The larger the surface area, the faster the liquid evaporates. The warmer the liquid is, the faster it evaporates. Thus, surface area and temperature affect the rate of evaporation of a liquid.
Evaporation takes places from the surface of the liquid. The larger the surface area, the faster the liquid evaporates. The warmer the liquid is, the faster it evaporates. Thus, surface area and temperature affect the rate of evaporation of a liquid.
When a liquid is heated, its molecules get more energy and move faster. They knock into each other violently and bounce further apart. As the heating goes on, its molecules vibrate even faster. Bubbles of gas (due to air dissolved in water) appear inside the liquid. The whole process is called boiling. The temperature at which a liquid boils is called the boiling point.
The
molecules at the surface of the liquid gain enough energy to overcome the forces holding them together. They break away from the liquid and form a gas (vapour). As more of the liquid molecules escape to form a
gas, a liquid is said to evaporate. This occurs at the boiling point of a
liquid.
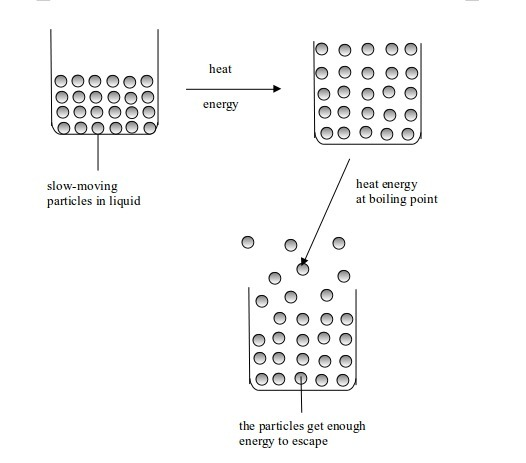
Change in state from liquid to gas
The
the temperature at which a liquid boils explains how strong the forces
holding its particles (molecules) together are. Liquids with high
boiling points have strong forces of attraction between their molecules
than those liquids with low boiling points.
The
the boiling point of a liquid can change if the surrounding pressure changes. If the surrounding pressure falls, the boiling point also falls. The boiling point of water at standard pressure (760 mmHg) is
100°C. On a high mountain, where pressure is low, it is lower than
100°C. If the surrounding pressure is increased, the boiling point rises. The same behaviour is experienced by gas when the pressure is either increased or decreased.
Condensation and solidification
The reverse of evaporation is condensation. This is brought about by cooling. When a gas is cooled down, its particles lose energy. They move more and more slowly. When they knock into each other, they do not have enough energy to bounce away again. They stay close together and
liquid forms. This process is called condensation. When the liquid is cooled further, the movement of the particles slows down even more. Eventually, they stop moving and solid forms. This is called solidification.
Condensation can be defined as a change in the state of a substance from gas (vapour) to liquid. Solidification is a change from liquid to a solid state of a substance. Solidification is the same as freezing.
Sublimation
A
few solids do not melt when they are heated. Instead, they change directly from the solid to a gaseous state without passing through the liquid state. This change in state is called sublimation. When a
solid changes directly into gas, it is said to sublime. Iodine, solid carbon dioxide ("dry ice") and ammonium chloride are examples of solids that sublime. Like melting, sublimation also occurs at one particular temperature for each pure solid.
Explain the importance of changing one state of matter to another
The following points summarize the importance of change in the state:
1. Separation of mixtures
Different
mixtures can be separated through such processes as distillation,
sublimation, evaporation and condensation. Let us have a look at an example of distillation. This process involves boiling, evaporation and condensation. Distillation is a process can be applied in the separation of a
mixture (solution) of two or more substances. A mixture of two or more substances with different boiling points e.g. water and alcohol can be separated by this means. In such a case, a container with the mixed-up
liquids are heated. The liquid with a low boiling point evaporates and condenses first, leaving the one with a high boiling point in the container. The distillate (liquid with the low boiling point) is collected,
cooled down and transferred into another container.
2. Industrial manufacture of products
Industrially,
the process of distillation is applied in the production of pure substances such as beer and other alcoholic drinks such as wine, vodka, konyagi, etc. The manufacturing process involves boiling, evaporating and condensation.
3. Refining of petroleum (crude oil)
Crude
the oil contains organic liquid components, each with a different boiling point. In the refinery, the components with lower boiling points evaporate first and get separated out, leaving those with higher boiling points behind. In this way, we get various types of oil components
(fractions) such as petrol, diesel, kerosene, lubricating oil, etc.
4. Drying of crops and clothes
When
you suspend your clothing on a cloth line to dry, the moisture in it is lost through evaporation. Likewise, farmers in the village often spread crops on the ground to dry. They do this in order to reduce moisture content and hence prevent decaying. The moisture contained in crops leaves by evaporation. Therefore, you can notice how evaporation, as a
change in state, is important in everyday lives.
5. Cooling of our bodies in hot weather
You
all like to drink cold water or beverages, especially during hot weather. You can use a refrigerator to cool down drinking water or beverages directly. Alternatively, you can freeze water into ice and then use the resulting ice for cooling the beverage. Ice blocks are also saleable. Moreover, one can earn some money if she freezes water into ice blocks and then sells them to beverage vendors. Perishable products such as fish, meat, milk, etc are often packed in ice blocks to prevent them from going bad. Ice, as we studied early, is formed when water freezes (a change in state from liquid to solid).
6. Ice formation in refrigerators
You
all like to drink cold water or beverages, especially during hot weather. You can use a refrigerator to cool down drinking water or beverages directly. Alternatively, you can freeze water into ice and then use the resulting ice for cooling the beverage. Ice blocks are also saleable. Moreover, one can earn some money if she freezes water into ice blocks and then sells them to beverage vendors. Perishable products such as fish, meat, milk, etc are often packed in ice blocks to prevent them from going bad. Ice, as we studied early, is formed when water freezes (a change in state from liquid to solid).
7. Melting metals to make alloys
In
metallurgical industries, the need may arise to mix two or more metals
(alloys) together. This is only possible, where two or more metals are first melted at high temperatures into liquids. Then the resulting liquid metals are mixed in appropriate proportions. This is followed by cooling down the mixture to a solid alloy. Normally alloys have better qualities than individual metals.
8. Testing the purity of substances
The
presence of impurity may lower or raise the boiling point of the substance. A pure substance melts and boils at definite temperatures . The values for the melting point and boiling point are precise and predictable. This means that we can use them to test the purity of a sample. They can also be used to check the identity of
an unknown substance.
A typical example
Sea
water is impure. It freezes at a temperature well below the freezing point of pure water (0°C) and boils at a temperature above the boiling point of pure water (100°C). Other substances behave in a similar manner. So, boiling as a change in state can be used to test for the purity of a substance.
In
addition, the impurity also reduces the exactness of the melting or boiling point. An impure substance melts or boils over a range of temperature, not at a particular point.
9. Formation of rain
Perhaps
the most important of all, as far as change in state is concerned, is
the formation of rain. Rained is mainly formed through the process of
evaporation and condensation. Water vapour, evaporating mostly from
water bodies (oceans, seas, lakes, rivers, ponds, etc), land and plants
rises up to the sky. As it rises, it cools down and condenses into tiny
droplets
On
further cooling as they rise up, these droplets form bigger water
drops. Owing to gravitational force, these drops fall down as rainfall.
Every one of you knows how important rain is to our life. Therefore, you
have noticed how evaporation and condensation, as changes in state,
contribute to rain formation.
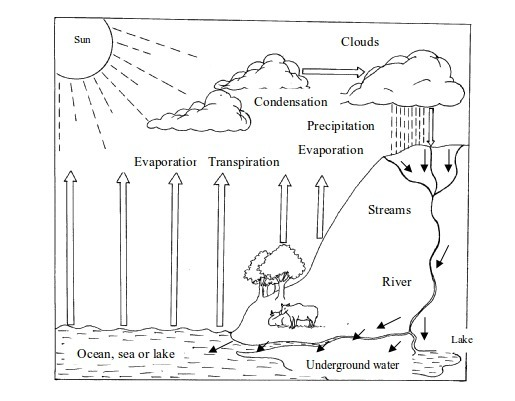
Rain formation
Kinetic nature of matter
We
already know that matter is composed of atoms, ions or molecules. We
have not yet considered the reason why the same substance, say water,
can exist in more than one form, for example as solid ice, liquid water,
and gaseous steam. But does matter behave like that?
The kinetic theory of matter has been used to explain the way in which the arrangement of the particles of a substance can determine the properties of that substance, and particularly the state in which it is likely to be found under a given set of conditions. The idea is that all matter is made up of tiny moving particles. The main points of the theory are as follows:
- All matter is made up of tiny particles (atoms and molecules) that are invisible to the naked eye and to most microscopes.
- The particles are moving all the time. The higher the temperature is, the higher the average energy of the particles.
- Heavier particles move more slowly than lighter particles at the same temperature.
- Each substance has unique particles that are different from the particles of other substances.
- The particles of matter are held together by strong electrostatic forces.
- There are empty spaces between the particles of matter that are very large compared to the particles themselves.
The solid state
In
the solid state, the particles are so closely packed (see figure
bellow. The particles are held together by strong forces of attraction
that act like a chemical glue. Free movement of particles cannot take
place. They cannot move around freely in this arrangement. Instead, they
vibrate about a fixed position. They are arranged in a fixed pattern
which form a cluster of vibrating masses. This makes a solid to have a
fixed shape, which cannot be changed except by applying strong external
forces.
The liquid state
The
particles of a liquid are also closely packed but the forces of
attraction between them are weaker than of a solid. These forces of
attraction tend to bind them together. The particles have more kinetic
energy and they can move around each other. The binding forces are
strong when particles come close to one another. It is thought that the
particles of a liquid are fairly randomly arranged but consist of
"clusters" closely packed together. This property makes a liquid to have
a definite volume. However, since the particles are fairly free to move
a liquid does not have any characteristic shape (see figure 5.5(b).
Thus, a liquid will always take the shape of its container.
The gaseous state
The
gaseous state is one in which the particles are moving independently of
each other in all directions and at great speeds. The particles of a
gas are relatively far apart. They exert no force of attraction on each
other. They have more energy than the particles of solids and liquids.
They move rapidly and randomly, colliding with each other and with the
walls of the container. A typical speed for a molecule of hydrogen in
air at ordinary temperature and pressure has been found to be
approximately 500 ms-1
It
has been estimated that a nitrogen molecule makes collisions each
second. Thus, a gas will rapidly spread out to fill any container in
which it is placed. A gas cannot have any shape of its own.
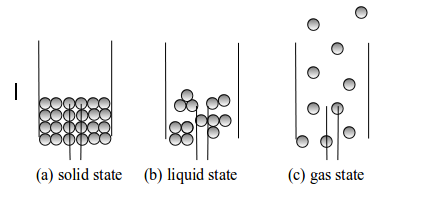
Three states of matter
Physical and chemical changes
Depending on the nature of change, all changes that matter undergoes can be classified as either physical or chemical.
The Characteristics of a Physical Change
Describe the characteristics of a physical change
Physical change
Substances
may undergo changes in their physical properties e.g. changes in
colour, shape (or form), state, density, structure and texture, etc. If
you take a stone and break it down into small particles, you will have
only changed its form, but it will remain as a stone. Likewise, melting
ice to water or freezing water to ice does not change it, but it is
still water. The same case happens when you dissolve salt in water to
get a solution of salt in water. You can still get back the original
salt by evaporation, except that the crystals of the salt obtained will
not look exactly the same as those of the original salt.
These changes of state are examples of physical changes. Physical changes such as melting and boiling do not result in new substances being formed
For example, ice and water still contain the same particles whether in solid (ice) liquid (water) or gaseous (vapour) state.
Changes in states
Characteristics
In
the explanation above, we find that in a physical change it is only the
physical form, and not the actual nature, of a substance that changes.
The changes are brought about by a mere addition or removal of heat, as
in the case with water or ice. Such a change is called a physical
change. It can be distinguished by the following characteristics:
- There is no formation of a new substance. Consider an example given above. The ice, liquid water and steam are the solid, liquid and gaseous forms of the same substance (water).
- There is no change in weight of the substance undergoing the change. If you start with 50g of ice, you will still get the same mass of water and steam (vapour) upon melting and boiling respectively.
- The changes are readily reversible. You can easily change water back to ice and vapour to water by a mere subtraction of heat (cooling).
- It is not accompanied by a great heat change. Just a little heat is required to change ice to water, and water to steam.
Physical Changes of Matter Experimentally
Demonstrate physical changes of matter experimentally
Experiment
- Add some common salt (sodium chloride) to distilled water in a beaker. Stir the mixture until the salt disappears and forms a solution with water. Transfer the water into a porcelain dish. Heat the content until all the water has evaporated off. The salt reappears in its original white solid form.
- Grind some roll sulphur in a mortar to powder. Put the resultant powder in a test-tube and heat gently, shaking all the time. The sulphur melts to an amber-coloured liquid. On cooling, this liquid returns to its original condition as a yellow solid.
- Put a block of ice in a beaker. Heat gently until the whole block melts to form water. Pour the water formed in a cup and place it in a deep freezer overnight. The water will freeze back to ice.
You
will have seen that all the above changes involve only changes in
physical forms of the substances. The chemical nature of substances
remained unchanged. Therefore, we can define a physical change as a
change that does not involve formation of a new substance but involves a
change in state or physical form of the substance and that such a form
can be reversed.
The Characteristics of a Chemical Change
Describe the characteristics of a chemical change
Chemical change
Some
changes that materials undergo are permanent. Such changes usually
involve changes in chemical properties of a substance. For example, when
you burn a piece of wood in fire, you get ash. The properties of wood
and ash are very different. There is no way you can change ash back to
wood. It is practically impossible. A permanent change in chemical
properties of a substance is called a chemical change. In a chemical
change, a substance losses all its physical and chemical properties.
Characteristics
Includes
- A chemical change results in the formation of a new substance. The new substance has different chemical and physical properties as compared to the original substance.
- It is generally not reversible. For example, you cannot turn the ash back to wood.
- There is a change in weight or mass of the substance undergoing the change. When you burn wood weighing 5 kg, you cannot expect to get the same weight of ash.
- The change is accompanied by a considerable heat change. For wood to burn to ash a lot of heat must be supplied.
Chemical Changes of Matter Experimentally
Demonstrate chemical changes of matter experimentally
Experiment
- Strongly heat some roll sulphur on a deflagrating spoon until it melts and begins to burn with a blue flame. If you continue heating, it gradually decreases in amount and finally the spoon will be left empty. The disappearance of sulphur is due to the formation of a new gaseous substance that is invisible. The presence and existence of a gas in air can be defected by its irritating smell. The gas can also be detected by burning the sulphur in a gas jar to which some blue litmus solution has been added. The gas formed, sulphur dioxide, will turn the blue litmus paper into a red one.
- With the aid of tongs, subject a piece of magnesium ribbon to a Bunsen burner flame. The ribbon burns to produce a new substance, white ash of magnesium oxide.
- Wrap a wet cotton wool around an iron nail. Keep it in a test tube for 3 days. By the 3rd day, some brown marks of rust will appear on the surface of the nail. Rust is hydrated iron (III) oxide. This is quite a new substance compared to iron nails.
Chemical and physical changes of matter
Depending on the nature of change, all changes that matter undergoes can be classified as either physical or chemical.
The Characteristics of a Physical Change
Physical change
Substances
may undergo changes in their physical properties e.g. changes in
colour, shape (or form), state, density, structure and texture, etc. If
you take a stone and break it down into small particles, you will have
only changed its form, but it will remain as a stone. Likewise, melting
ice to water or freezing water to ice does not change it, but it is
still water. The same case happens when you dissolve salt in water to
get a solution of salt in water. You can still get back the original
salt by evaporation, except that the crystals of the salt obtained will
not look exactly the same as those of the original salt.
These
changes of state are examples of physical changes. Physical changes
such as melting and boiling do not result in new substances being formed
For example, ice and water still contain the same particles whether in solid (ice) liquid (water) or gaseous (vapour) state.

Changes in states
Characteristics
In
the explanation above, we find that in a physical change it is only the
physical form, and not the actual nature, of a substance that changes.
The changes are brought about by a mere addition or removal of heat, as
in the case with water or ice. Such a change is called a physical
change. It can be distinguished by the following characteristics:
- There is no formation of a new substance. Consider an example given above. The ice, liquid water and steam are the solid, liquid and gaseous forms of the same substance (water).
- There is no change in weight of the substance undergoing the change. If you start with 50g of ice, you will still get the same mass of water and steam (vapour) upon melting and boiling respectively.
- The changes are readily reversible. You can easily change water back to ice and vapour to water by a mere subtraction of heat (cooling).
- It is not accompanied by a great heat change. Just a little heat is required to change ice to water, and water to steam.
Physical Changes of Matter Experimentally
Demonstrate physical changes of matter experimentally
Experiment
- Add some common salt (sodium chloride) to distilled water in a beaker. Stir the mixture until the salt disappears and forms a solution with water. Transfer the water into a porcelain dish. Heat the content until all the water has evaporated off. The salt reappears in its original white solid form.
- Grind some roll sulphur in a mortar to powder. Put the resultant powder in a test-tube and heat gently, shaking all the time. The sulphur melts to an amber-coloured liquid. On cooling, this liquid returns to its original condition as a yellow solid.
- Put a block of ice in a beaker. Heat gently until the whole block melts to form water. Pour the water formed in a cup and place it in a deep freezer overnight. The water will freeze back to ice.
You
will have seen that all the above changes involve only changes in
physical forms of the substances. The chemical nature of substances
remained unchanged. Therefore, we can define a physical change as a
change that does not involve formation of a new substance but involves a
change in state or physical form of the substance and that such a form
can be reversed.
The Characteristics of a Chemical Change
Chemical change
Some
changes that materials undergo are permanent. Such changes usually
involve changes in chemical properties of a substance. For example, when
you burn a piece of wood in fire, you get ash. The properties of wood
and ash are very different. There is no way you can change ash back to
wood. It is practically impossible. A permanent change in chemical
properties of a substance is called a chemical change. In a chemical
change, a substance losses all its physical and chemical properties.
Characteristics
Includes
- A chemical change results in the formation of a new substance. The new substance has different chemical and physical properties as compared to the original substance.
- It is generally not reversible. For example, you cannot turn the ash back to wood.
- There is a change in weight or mass of the substance undergoing the change. When you burn wood weighing 5 kg, you cannot expect to get the same weight of ash.
- The change is accompanied by a considerable heat change. For wood to burn to ash a lot of heat must be supplied.
Chemical Changes of Matter Experimentally
Demonstrate chemical changes of matter experimentally
Experiment
- Strongly heat some roll sulphur on a deflagrating spoon until it melts and begins to burn with a blue flame. If you continue heating, it gradually decreases in amount and finally the spoon will be left empty. The disappearance of sulphur is due to the formation of a new gaseous substance that is invisible. The presence and existence of a gas in air can be defected by its irritating smell. The gas can also be detected by burning the sulphur in a gas jar to which some blue litmus solution has been added. The gas formed, sulphur dioxide, will turn the blue litmus paper into a red one.
- With the aid of tongs, subject a piece of magnesium ribbon to a Bunsen burner flame. The ribbon burns to produce a new substance, white ash of magnesium oxide.
- Wrap a wet cotton wool around an iron nail. Keep it in a test tube for 3 days. By the 3rd day, some brown marks of rust will appear on the surface of the nail.
Rust is hydrated iron (III) oxide. This is quite a new substance
compared to iron nails.
Differences between physical changes and chemical changes of matter

Concept of compounds and mixtures
A
compound Is a substance that contains two or more elements chemically
combined together. A mixture is something that contains two or more
elements not combined chemically. It is always difficult to identify a
mixture from a compound. Before going any further into this topic, let
us start by looking at the differences between compounds and mixtures.
These differences are summarized in the table below.
The Concept of a Mixture
A
mixture is something that contains two or more substances not combined
chemically. The substances may mix up completely or they may remain
separate.
Our
environment is a mixture of all forms of matter. For example, the
earth's crust is a mixture of soils, rocks, minerals, and water. Sea,
river, and lake waters contain dissolved gases, living organisms and,
sometimes, salt. Air consists of gases, water vapour, and dust
particles. The components of each of these mixtures could be elements
such as oxygen, nitrogen, sulphur or gold. Alternatively, the mixture
might consist of elements and compounds such as hydrocarbons (e.g.
petroleum), water, metallic oxides or salts.
Other
substances that can form mixtures when placed or mixed together include
sand and sugar, maize and bean seeds, soil and table salt, water and
mud, etc.
Mixtures into Solutions, Suspensions and Emulsions
Classification of mixtures
Mixtures can be classified as solutions, suspensions or emulsions. This classification is based on whether the mixed substances dissolve completely or not. It also depends on the nature of the mixtures that result upon mixing. Let us look at each category in detail.
Solutions
A
solution is a uniform mixture of two or more substances. Such mixtures
may be a solid in a liquid, a liquid in a liquid, a liquid in a gas and,
very rarely, a gas in a gas. (See table bellow). We most often think
of a solution as being made of a solid dissolved in a liquid. For
example, solutions of sugar or salt in water are quite common. A solid
that dissolves in a liquid is called a solute while the liquid in which that solid dissolves is called a solvent. For example, sugar and salt are solutes and water is a solvent.
Types of solution
There are three types of solution namely:
Unsaturated solution
This is the one that can still dissolve more solute at a given temperature.
Saturated solution
This is the one that can dissolve no more solute at a given temperature.
Supersaturated solution
This is the one that temporarily hold more solute than the saturated solution at a given temperature
Application of saturation
The concept of saturation can be applied when
i. Separating certain mixture in the laboratory
ii. Extracting some minerals such as common salt
Suspensions
A
suspension is a cloudy mixture of solid particles suspended in a
liquid. A solid is said to be in suspension in a liquid when small
particles of it are contained in a liquid, but are not dissolved in it.
If the mixture is left undisturbed, the solid particles will slowly
settle to the bottom of the containing vessel, leaving the pure liquid
above them.
Muddy
water is a typical suspension. The mud would settle after a time if
left undisturbed leaving brown residue on the bottom of the containing
vessel and clear water above. The particles of mud would be retained by
filtering whilst the water (and any solids in solution) would pass
through.
If
you mix flour or chalk dust in water, it forms a suspension. Their
particles are simply dispersed (spread) throughout the water and would
eventually settle down to the bottom of the vessel if left undisturbed
for sometime.
Main Difference – Solution vs Suspension
Solutions and suspensions are both considered as mixtures. The key difference between solution and suspension is their particle size. Particles in a solution are much smaller than that of suspensions. Due to this difference between solute particles and suspension particles, there are distinct differences in the two systems. However, the components of both the systems are not chemically bonded to each other and can be separated based on their physical properties such as size, solubility and density.
Emulsions
An
emulsion is a cloudy mixture of tiny droplets of one liquid suspended
in another liquid. Sometimes two immiscible liquids will not separate
out into two layers when mixed together. One of the liquid may form
droplets and spread throughout the other to form an emulsion.
Cooking oil and water do not mix but they will form an emulsion when
they are mixed and shaken. Droplets of oil will spread throughout the
water. Unlike pure liquids, emulsions are cloudy (opaque). So you cannot
see through them. The emulsion will not settle like a suspension. Which
other liquids you know can form suspensions?
Formation of mixtures
Mixtures can be formed from different substances in two major ways.
The first type constitutes homogenous mixtures, where the substances are totally mixed together uniformly. Examples include solutions of salts and sugars in water.
The second type constitutes heterogeneous mixtures,
where the substances remain separate and one substance is spread
throughout the other as small particles, droplets, or bubbles. All
emulsions and suspensions fall under this category. Examples include
suspensions of insoluble solids or oil droplets in water.
Separation of mixtures
To
make use of the materials around us, we need methods for physically
separating the many and varied mixtures that we come across. One of the
distinctive characteristics of a mixture of substances is that it is
usually possible to separate the constituents by physical means. There
are many different physical methods used to separate a wide variety of
mixtures. The particular method employed to separate any given mixture
depends upon the nature of its constituents. The following are some of
the methods in wide use.
The Different Methods of Separating Mixtures
1. Filtration
This method is best applicable in separation of components of mixtures called suspensions.
A
mixture of chalk dust or flour with water can be separated by filtering
the suspension. The suspended particles get trapped in the filter
paper. The trapped particles are called the residue. The water is called the filtrate
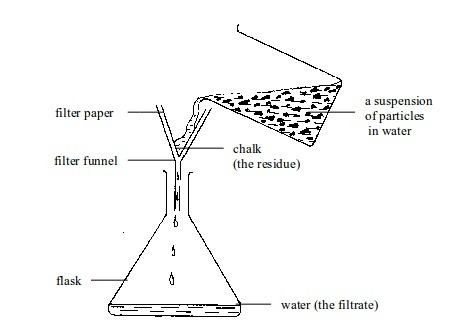
Filtration
2. Decantation
This
is another method that can be used to separate mixtures called
suspensions. However, in this case, separation will be successful if the
suspended particles are large enough. Otherwise, the decantation
exercise should be accompanied by filtration if you want to get a clear
liquid.
Once the solid has settled to the bottom of the container (sedimented), the liquid can be carefully poured off. This is called decantation. Decantation can be applied to separate such components as mixtures of mud, sand or gravel in water and so on.
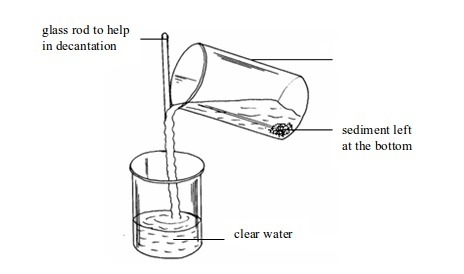
Decantation of muddy water
3. Evaporation
This
method is used to separate substances that form a solution. In such a
mixture, the solute is completely dissolved in a solvent to make a
uniform solution. To separate these substances, the solution is heated
so that the solvent evaporates, leaving the solid residue behind.
A mixture of salt or sugar in water can be separated by applying this method.
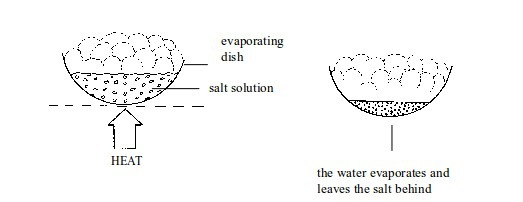
Evaporating the solvent
4. Simple distillation
Separating
a liquid from a solution can be carried out by distillation. The
boiling point of a liquid is usually very much lower than that of the
dissolved solid. The liquid can easily be evaporated off in a
distillation flask. It is condensed by passing it down a water-cooled
condenser and then collected as the distillate
This
method can be used to obtain pure water from impure water or from water
with dissolved impurities. The process may be used to separate a liquid
from a solution or to separate two liquids whose boiling points differ
by an appreciable temperature interval. This is a way of getting a pure
solvent out of a solution.
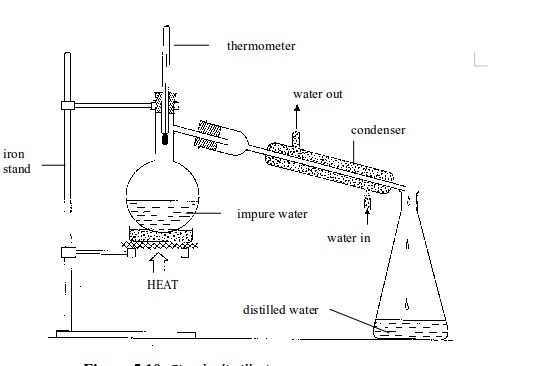
Simple distillation
5. Fractional distillation
Separating
the liquids from a mixture of two (or more) miscible liquids is again
based on the fact that liquids will have different boiling points.
However, the boiling points are closer together than for solid-in-liquid
solutions. It is difficult to separate mixtures of liquids whose
boiling points differ by only a few degrees. In this case, fractional
distillation is used.
For
example, ethanol boils at 78°C whereas water boils at 100°C. When a
solution of ethanol and water is heated, ethanol and water vapours
enters the fractionating column. Evaporation and condensation take place
as the vapours rise up the column. Ethanol passes through the condenser
first as the temperature of the column is raised above the boiling
point. Water condenses in the column and flows back into the flask
because the temperature of the column is below its boiling point of
100°C.
The
temperature on the thermometer stays at 78°C until the ethanol has
distilled over. Eventually, the thermometer reading rises above 78C°.
This is a sign that all the ethanol has been separated, so heating can
be stopped. By watching the temperature carefully, the two liquids
(fractions) can be collected separately.
Various
forms of fractionating column can be used. Their general purpose is to
provide surfaces, e.g. flat discs, on which ascending vapour can
condense. Glass beads in the column provide a large surface area for
condensation.
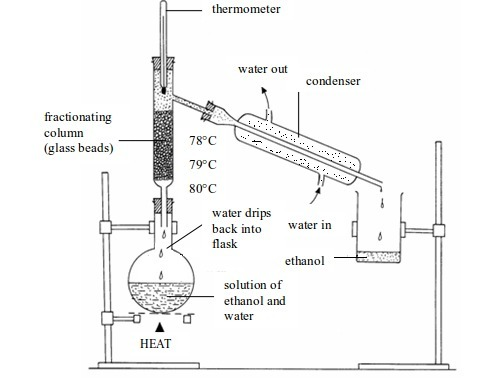
Fractional distillation
6. Sublimation
This
is a technique used to separate a mixture of solids where one of the
solids sublimes. Examples of solids which sublime are ammonium chloride,
iodine, solid carbon dioxide and naphthalene. A mixture of any of these
solids with another solid can be separated by sublimation.
Let
us consider a mixture of iodine and sodium chloride. The mixture is
placed in a beaker and covered with a filter funnel as shown in the
diagram below. Then, as the mixture is heated, the ammonium chloride
sublimes. The ammonium chloride vapour rises and condenses on the cooler
walls of the filter funnel. The sodium chloride is left in the beaker.
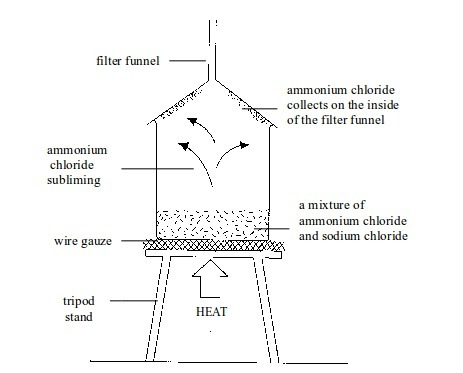
Sublimation of ammonium chloride
7. Chromatography
This
method is commonly used to separate a mixture of coloured substances
(solids or dyes). An example of this is the separation of dyes that make
up black ink. Chromatography works better when a solvent is used. The
commonest solvent is water, though other solvents such as ethanol or
ether may be used for those substances that do not dissolve in water.
There are two types of chromatography, namely column chromatography and paper chromatography.
The two types of chromatography follow the same principle, but paper
chromatography is the simplest form to set up, and hence is more
commonly used. On which principle does chromatography work? Let us
consider an example of separating dyes that make up black ink. In this
case, water is used as a solvent.
Separating dyes in ink
Procedure
- Put a small spot of the water-soluble ink onto a strip of filter paper as shown in figure bellow
- Place the filter paper in a beaker of water. Make sure the level of the water is below the level of the ink spot.
- Leave the filter paper until the water has risen to the top of the paper.
- Remove the paper and allow it to dry.
- Note the colours the ink contains.
Observation
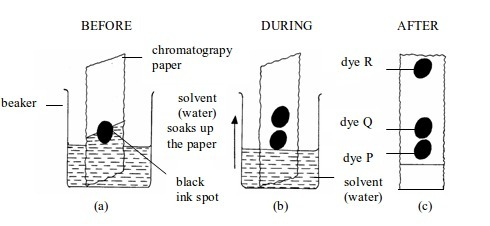
Separating the components of black ink by paper chromatography
As
the solvent (water) moves up the paper, the dyes are carried with it
and begin to separate. They separate because they have different
solubilities in water and are absorbed to different degrees by the
filter (chromatography) paper. As they rise, they are gradually
separated.
Findings
The
different colours of the ink make a pattern of colours formed during
the process of chromatography. This pattern of colours is called a
chromatogram.
Figure
aboveshows a chromatogram of black ink. The blue ink has the fastest
speed. This means it is the most soluble in water and least absorbed by
the paper. The green ink has travelled least. This means it is the least
soluble in water and most absorbed by the paper.
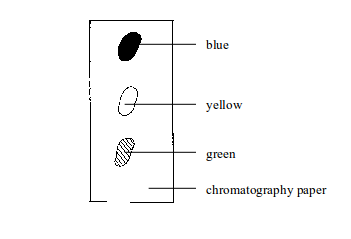
Paper chromatography showing the separated components of black ink
Uses of Chromatography
Chromatography is used in many different ways. The following are some of the application of chromatography:
- It can be used to find out the components of a liquid or solid, or even to identify different substances.
- It can be used by security agents and medical personnel to analyse blood and urine samples.
- Causes of pollution in water and in animals that live in water can also be detected using chromatography.
- In chemistry, chromatography is used to test the purity of substances and in separation of mixtures.
8. Layer separation
Mixtures
of two immiscible liquids can be separated with a separating funnel.
The mixture is placed in a separating funnel and allowed to stand. The
liquids separate into two different layers. The lower denser layer is
then "tapped" off at the bottom.
For
example, when a mixture of kerosene and water is poured into the
funnel, the kerosene floats to the top as shown in figure above. When
the tap is opened, the water runs out. The tap is closed again when all
water has gone, leaving the kerosene in the funnel.
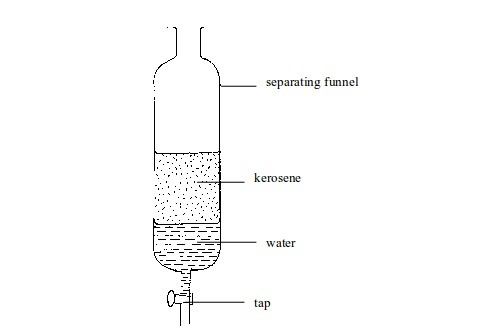
Separating immiscible liquids
9. Solvent extraction
Solvent
extraction, also known as liquid-liquid extraction, refers to the
separation of materials of different chemical types and solubilities by
selective solvent extraction. That is, some materials are more soluble
in one solvent than in another. The method is used to refine petroleum
products, chemicals, vegetable oils, and vitamins.
This
method is used is to separate a solid from a solution in which there is
more than one solid dissolved. An example of this is a water solution
of iodine and sodium chloride.
EXPERIMENT:Separating iodine from sodium chloride by solvent extraction.
Method
- Put the solution into a separating funnel as shown in figure (a).
- Add ethoxyethane. This forms a layer on top of the solution (b). The ethoxyethane is called the extracting solvent.
- Stopper the separating funnel and shake well figure (c). The iodine, which is more soluble in the ethoxyethane, passes into the ethoxyethane layer. The sodium chloride remains in the water layer.
- The water layer is run off into a beaker followed by the ethoxyethane layer into another beaker (Caution: Remove the stopper before opening the tap).
- The ethoxyethane is then evaporated off by simple distillation. Similarly, the water layer can be evaporated to yield sodium chloride.
The solvent extraction works on two principles:
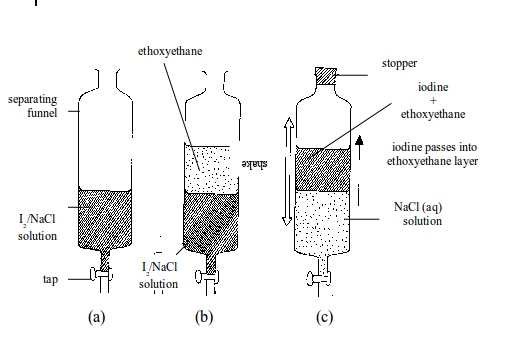
Separating iodine from sodium chloride by solvent extraction
- One solid in the solution must be more soluble in the extracting solvent than the other.
- The extracting solvent must not be miscible with the solvent in which the mixture of solids is dissolved. Neither should it react with it.
10. Centrifugation
A
centrifuge is used to separate small amounts of suspension.
Centrifugation is used with insoluble solids where the particles are
very small and spread throughout the liquid. In centrifugation, test
tubes containing suspensions are spun round very fast. The solid gets
thrown to the bottom. Here, it is no longer the force of gravity on the
solid that causes settling.
Instead,
there is a huge centrifugal force acting on the particles due to the
high speed spinning of the samples. This causes the solid to be
deposited at the bottom of the centrifuge tube.
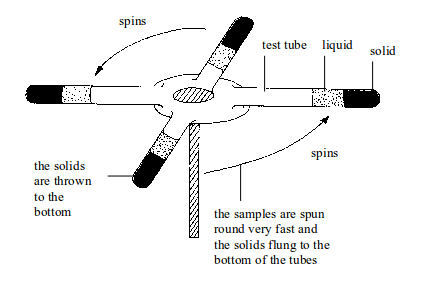
Separation by centrifugation
After centrifugation, the liquid can be decanted (poured out) from the test tube, or removed with a small pipette.
This makes the solid to be left behind.
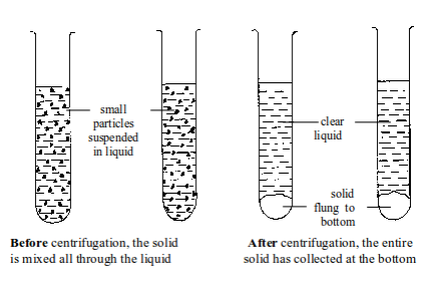
The solid after centrifugation
11. Magnetic separation
If
the solid mixture contains iron, the iron can be removed using a
magnet. This method is used to separate scrap iron from other metals.
Magnetic iron ore can be separated from other material in the crushed
ore by using an electromagnet. In the process of recycling metals, iron
objects can be picked out from other scrap metals using electromagnets.
12. Crystallization
This
process involves evaporation but the speed of evaporation is much
slower. In principle the salt solution can be left in the evaporating
basin for a long period until all the water has evaporated but in
practice this takes longer time. The process begins by evaporating away
the liquid. However, because the crystals are needed, evaporation is
stopped after the solution has been concentrated enough. The
concentrated solution is allowed to cool slowly and crystallize. The
crystals so formed can be filtered off and dried. A similar process is
used to extract salt from the sea. Salty sea water is placed in wide
basins and put in the sun. Water evaporates off, leaving the salt
crystals in basins.
13. Winnowing or threshing
This
is a method used to separate grains from husks or bran. The process
makes use of the differences in density of the constituents in the
mixture. When the winnower is shaken around, grains, being denser than
husks or bran, sink to the bottom of the winnower
The
less dense husks or bran moves to the top. They are then blown off the
winnower by wind or breath, or sometimes picked by hand and separated
from the grains.

Separating thresh from grains by winnowing
The Significance of Separating Different Mixtures
We
separate mixtures in order to obtain the mixture constituents and put
them into appropriate use. The world around us is made up of mixtures of
different substances. These substances are often of little use when
they are in the form of a mixture. For this reason, separation of the
individual components of mixtures is deemed inevitable.
1. (i) Fractional distillation
Is
used industrially to separate the various fractions of crude oil such
as natural gas, petrol, kerosene, diesel, lubricating oils, waxes,
asphalt and bitumen. All these fractions have a significant use in man’s
industrial, domestic and commercial activities. The functions or use of
all these fractions is well known to everyone of us. Can you mention
the functions of each fraction? You will learn more about these products
in organic chemistry section.
(ii) Fractional distillation of liquid air
Separates
the air into its component gases. This is important because these
components have many uses in our everyday life. Some of these fractions
and their functions are summarized below:
| Component | Use |
| Nitrogen | Manufacture of fertilizer |
| Oxygen | Used in hospitals, steel making, diving and space travel |
| Argon | Filling light bulbs |
| Carbon dioxide | Fire extinguishing, used in carbonate drinks, etc. |
| Helium | Filling airships and water balloons |
| Krypton and Xenon | Used in photographic flash lamps |
2. Filtration and purification of drinking water make use of processes such as decantation, filtration and sometimes distillation. The bottled water we drink is prepared by some or a combination of these processes.
3. In mining, an electromagnet is used to separate magnetic iron ore from other materials in the crushed ore.
4. In the manufacture of ethanol by fermentation in breweries, distillation is used in the final stage to purify ethanol to its purest form (surgical spirit) in which the ethanol is usually sold. Likewise, distillation of fermented starch (8-12% ethanol), yields alcoholic drinks called sprits (whisky, gin, brandy, rum) which contain about 35-40% ethanol.
5. (i) Paper chromatography is very useful in analysis of substances present in a solution. For example, it can tell whether a substance has become contaminated or otherwise. This can be very important, because contamination of food or drinking water, for instance, may be dangerous to our health.
(ii) Chromatography has
proved very useful in the analysis of biologically important molecules
such as sugars, amino acids, and nucleotide bases. Molecules such as
amino acids can be seen if the paper is viewed under ultra- violet
light.
(iii) Paper chromatography
is the test that can be used to check for the purity of a substance. If
the sample is pure, it should only give one spot when run in several
different solvents.
6. Other separation methods are also used to check whether purification has been successful. Samples obtained by distillation can be re-distilled. The purity of crystals can be improved by re-crystallisation. A water sample can be tested for amount of dissolved material by evaporating a certain amount of water to dryness. The solid waste can be weighed. This would give the amount of dissolved solid in the water.
The
process of purification is of crucial importance in many areas of
chemical industry. Medical drugs (pharmaceuticals) must be of highest
possible degrees of purity. Any contaminating substances even in very
small amounts may have harmful side effects.
7. (i) Separation of cream from whole milk is done by the process of centrifugation. As the milk is spun, the heavier contents are forced down and the lighter cream rises up. After centrifugation, the cream is poured off the top by decantation. This is the initial stage of milk constituent separation, after which other components such as milk proteins (cheese) are separated.
(ii)
Centrifugation is applicable in blood analysis, where the solid part of
blood is separated from the liquid part by centrifugation. Blood is a
suspension containing microscopic blood cells (corpuscles) in a liquid
called plasma. If blood is centrifuged in a test tube, the blood cells are flung to the bottom, leaving the liquid plasma on top.
8. Knowledge of separation of two immiscible liquids can be applied in the extraction of metals such as iron from their ores. For example, at the base of the blast furnace, the molten slug forms a separate layer on top of the liquid iron. The two can then be "tapped" off separately. The method is very useful in organic chemistry as part of the process called solvent extraction.
9. Evaporation process is used in the extraction of common salt from seawater whereby the sun evaporates water molecules from salty water, leaving crystals of the salt behind.
10. Layer separation technique is applied in the recovery of liquids from contaminants.
11. Solvent extraction process is applied in the extraction of certain edible oils from seeds, and in the extraction of some metals from sludge mixture.






